Diffuse interface models in fluid mechanics
Diffuse interface models in fluid mechanics
Diffuse interface models in fluid mechanics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
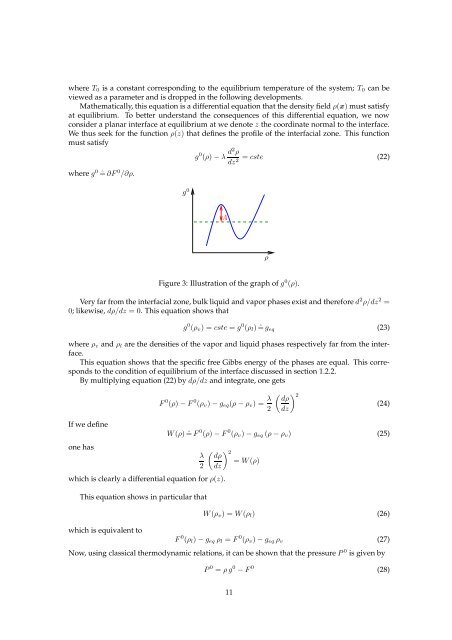
where T 0 is a constant correspond<strong>in</strong>g to the equilibrium temperature of the system; T 0 can beviewed as a parameter and is dropped <strong>in</strong> the follow<strong>in</strong>g developments.Mathematically, this equation is a differential equation that the density field ρ(x) must satisfyat equilibrium. To better understand the consequences of this differential equation, we nowconsider a planar <strong><strong>in</strong>terface</strong> at equilibrium at we denote z the coord<strong>in</strong>ate normal to the <strong><strong>in</strong>terface</strong>.We thus seek for the function ρ(z) that def<strong>in</strong>es the profile of the <strong>in</strong>terfacial zone. This functionmust satisfywhere g 0 ˆ= ∂F 0 /∂ρ.g 0g 0 (ρ) − λ d2 ρ= cste (22)dz2 AρFigure 3: Illustration of the graph of g 0 (ρ).Very far from the <strong>in</strong>terfacial zone, bulk liquid and vapor phases exist and therefore d 2 ρ/dz 2 =0; likewise, dρ/dz = 0. This equation shows thatg 0 (ρ v ) = cste = g 0 (ρ l ) ˆ= g eq (23)where ρ v and ρ l are the densities of the vapor and liquid phases respectively far from the <strong><strong>in</strong>terface</strong>.This equation shows that the specific free Gibbs energy of the phases are equal. This correspondsto the condition of equilibrium of the <strong><strong>in</strong>terface</strong> discussed <strong>in</strong> section 1.2.2.By multiply<strong>in</strong>g equation (22) by dρ/dz and <strong>in</strong>tegrate, one getsF 0 (ρ) − F 0 (ρ v ) − g eq (ρ − ρ v ) = λ 2( ) 2 dρ(24)dzIf we def<strong>in</strong>eone haswhich is clearly a differential equation for ρ(z).W (ρ) ˆ= F 0 (ρ) − F 0 (ρ v ) − g eq (ρ − ρ v ) (25)λ2This equation shows <strong>in</strong> particular thatwhich is equivalent to( ) 2 dρ= W (ρ)dzW (ρ v ) = W (ρ l ) (26)F 0 (ρ l ) − g eq ρ l = F 0 (ρ v ) − g eq ρ v (27)Now, us<strong>in</strong>g classical thermodynamic relations, it can be shown that the pressure P 0 is given byP 0 = ρ g 0 − F 0 (28)11










![[Diffusion-Limited Aggregation - A Model for Pattern Formation].](https://img.yumpu.com/52395246/1/190x245/diffusion-limited-aggregation-a-model-for-pattern-formation.jpg?quality=85)




