Therapeutics
Role of N-Acetylcysteine
Role of N-Acetylcysteine
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Role of N-Acetylcysteine in the treatment of Acute Respiratory Disorders<br />
8.1 Clinical Trials<br />
8.1.1 Effect on Influenza-like Symptoms<br />
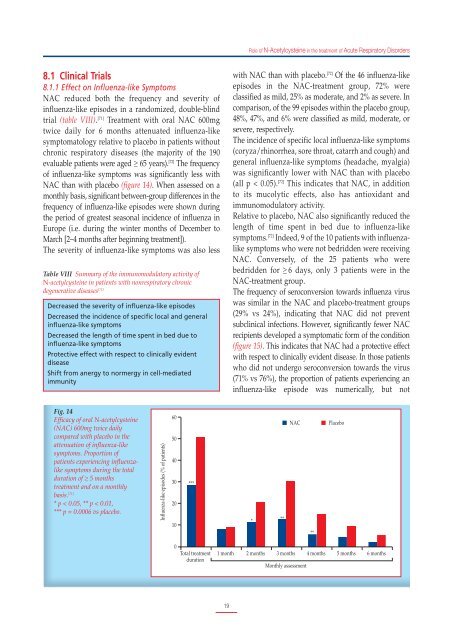
NAC reduced both the frequency and severity of<br />
influenza-like episodes in a randomized, double-blind<br />
trial (table VIII). [71] Treatment with oral NAC 600mg<br />
twice daily for 6 months attenuated influenza-like<br />
symptomatology relative to placebo in patients without<br />
chronic respiratory diseases (the majority of the 190<br />
evaluable patients were aged ≥ 65 years). [72] The frequency<br />
of influenza-like symptoms was significantly less with<br />
NAC than with placebo (figure 14). When assessed on a<br />
monthly basis, significant between-group differences in the<br />
frequency of influenza-like episodes were shown during<br />
the period of greatest seasonal incidence of influenza in<br />
Europe (i.e. during the winter months of December to<br />
March [2–4 months after beginning treatment]).<br />
The severity of influenza-like symptoms was also less<br />
Table VIII Summary of the immunomodulatory activity of<br />
N-acetylcysteine in patients with nonrespiratory chronic<br />
degenerative diseases [71]<br />
Decreased the severity of influenza-like episodes<br />
Decreased the incidence of specific local and general<br />
influenza-like symptoms<br />
Decreased the length of time spent in bed due to<br />
influenza-like symptoms<br />
Protective effect with respect to clinically evident<br />
disease<br />
Shift from anergy to normergy in cell-mediated<br />
immunity<br />
with NAC than with placebo. [72] Of the 46 influenza-like<br />
episodes in the NAC-treatment group, 72% were<br />
classified as mild, 25% as moderate, and 2% as severe. In<br />
comparison, of the 99 episodes within the placebo group,<br />
48%, 47%, and 6% were classified as mild, moderate, or<br />
severe, respectively.<br />
The incidence of specific local influenza-like symptoms<br />
(coryza/rhinorrhea, sore throat, catarrh and cough) and<br />
general influenza-like symptoms (headache, myalgia)<br />
was significantly lower with NAC than with placebo<br />
(all p < 0.05). [72] This indicates that NAC, in addition<br />
to its mucolytic effects, also has antioxidant and<br />
immunomodulatory activity.<br />
Relative to placebo, NAC also significantly reduced the<br />
length of time spent in bed due to influenza-like<br />
symptoms. [72] Indeed, 9 of the 10 patients with influenzalike<br />
symptoms who were not bedridden were receiving<br />
NAC. Conversely, of the 25 patients who were<br />
bedridden for ≥ 6 days, only 3 patients were in the<br />
NAC-treatment group.<br />
The frequency of seroconversion towards influenza virus<br />
was similar in the NAC and placebo-treatment groups<br />
(29% vs 24%), indicating that NAC did not prevent<br />
subclinical infections. However, significantly fewer NAC<br />
recipients developed a symptomatic form of the condition<br />
(figure 15). This indicates that NAC had a protective effect<br />
with respect to clinically evident disease. In those patients<br />
who did not undergo seroconversion towards the virus<br />
(71% vs 76%), the proportion of patients experiencing an<br />
influenza-like episode was numerically, but not<br />
Fig. 14<br />
Efficacy of oral N-acetylcysteine<br />
(NAC) 600mg twice daily<br />
compared with placebo in the<br />
attenuation of influenza-like<br />
symptoms. Proportion of<br />
patients experiencing influenzalike<br />
symptoms during the total<br />
duration of ≥ 5 months<br />
treatment and on a monthly<br />
basis. [71]<br />
* p < 0.05, ** p < 0.01,<br />
*** p = 0.0006 vs placebo.<br />
Influenza-like episodes (% of patients)<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
***<br />
* **<br />
NAC<br />
**<br />
Placebo<br />
0<br />
Total treatment<br />
duration<br />
1 month 2 months 3 months<br />
Monthly assessment<br />
4 months 5 months 6 months<br />
19