Adsorption Isotherm of Methylene Blue on KNbO3 Compound
Adsorption Isotherm of Methylene Blue on KNbO3 Compound
Adsorption Isotherm of Methylene Blue on KNbO3 Compound
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
78<br />
Analysis<br />
Crystal structure and microstructure <str<strong>on</strong>g>of</str<strong>on</strong>g> the<br />
samples were measured by using an X-ray<br />
diffractometer (XRD, JDX-03530, JEOL, Japan),<br />
and a scanning electr<strong>on</strong> microscope (SEM, JEM-<br />
2010, JEOL, Japan), respectively. Specific surface<br />
area was measured by means <str<strong>on</strong>g>of</str<strong>on</strong>g> nitrogen<br />
adsorpti<strong>on</strong> method (BET, Quantachrome Autosorb<br />
Automated Gas Sorpti<strong>on</strong>).<br />
Results and Discussi<strong>on</strong><br />
Figure 1 shows XRD patterns <str<strong>on</strong>g>of</str<strong>on</strong>g> the starting<br />
powder and the hydrothermal product. The starting<br />
powder showed the XRD pattern corresp<strong>on</strong>ding<br />
to the Nb2O5 phase (JCPDF # 37-1468). The<br />
hydrothermal product showed the XRD pattern<br />
corresp<strong>on</strong>ding to <strong>KNbO3</strong> phase (JCPDF # 71-2171).<br />
Intensity Intensity (a.u.) (a.u)<br />
0 10 20 30 40 50 60 70<br />
Figure 1. XRD patterns <str<strong>on</strong>g>of</str<strong>on</strong>g> the <strong>KNbO3</strong> product and<br />
starting Nb2O5 powder.<br />
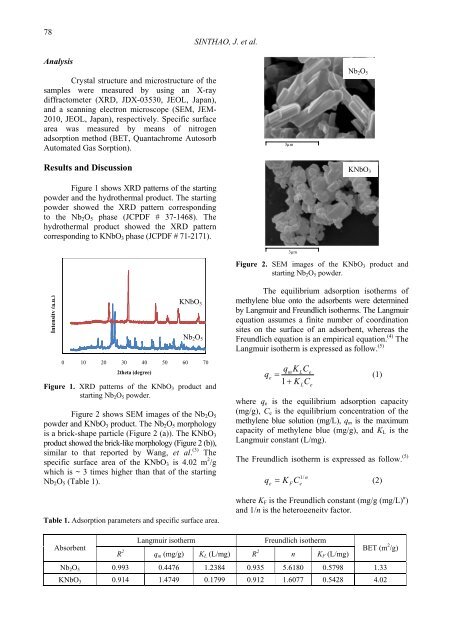
Figure 2 shows SEM images <str<strong>on</strong>g>of</str<strong>on</strong>g> the Nb2O5<br />
powder and <strong>KNbO3</strong> product. The Nb2O5 morphology<br />
is a brick-shape particle (Figure 2 (a)). The <strong>KNbO3</strong><br />
product showed the brick-like morphology (Figure 2 (b)),<br />
similar to that reported by Wang, et al. (3) The<br />
specific surface area <str<strong>on</strong>g>of</str<strong>on</strong>g> the <strong>KNbO3</strong> is 4.02 m 2 /g<br />
which is ~ 3 times higher than that <str<strong>on</strong>g>of</str<strong>on</strong>g> the starting<br />
Nb2O5 (Table 1).<br />
Absorbent<br />
2theta (degree)<br />
<strong>KNbO3</strong><br />
Nb2O5<br />
Table 1. <str<strong>on</strong>g>Adsorpti<strong>on</strong></str<strong>on</strong>g> parameters and specific surface area.<br />
SINTHAO, J. et al.<br />
Figure 2. SEM images <str<strong>on</strong>g>of</str<strong>on</strong>g> the <strong>KNbO3</strong> product and<br />
starting Nb2O5 powder.<br />
The equilibrium adsorpti<strong>on</strong> isotherms <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
methylene blue <strong>on</strong>to the adsorbents were determined<br />
by Langmuir and Freundlich isotherms. The Langmuir<br />
equati<strong>on</strong> assumes a finite number <str<strong>on</strong>g>of</str<strong>on</strong>g> coordinati<strong>on</strong><br />
sites <strong>on</strong> the surface <str<strong>on</strong>g>of</str<strong>on</strong>g> an adsorbent, whereas the<br />
Freundlich equati<strong>on</strong> is an empirical equati<strong>on</strong>. (4) The<br />
Langmuir isotherm is expressed as follow. (5)<br />
q<br />
e<br />
qmK<br />
LCe<br />
=<br />
1+<br />
K C<br />
L<br />
e<br />
(1)<br />
where qe is the equilibrium adsorpti<strong>on</strong> capacity<br />
(mg/g), Ce is the equilibrium c<strong>on</strong>centrati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the<br />
methylene blue soluti<strong>on</strong> (mg/L), qm is the maximum<br />
capacity <str<strong>on</strong>g>of</str<strong>on</strong>g> methylene blue (mg/g), and KL is the<br />
Langmuir c<strong>on</strong>stant (L/mg).<br />
The Freundlich isotherm is expressed as follow. (5)<br />
q<br />
e<br />
= K C<br />
(2)<br />
F<br />
1/<br />
n<br />
e<br />
where KF is the Freundlich c<strong>on</strong>stant (mg/g (mg/L) n )<br />
and 1/n is the heterogeneity factor.<br />
Langmuir isotherm Freundlich isotherm<br />
R 2 qm (mg/g) KL (L/mg) R 2 n KF (L/mg)<br />
Nb2O5<br />
<strong>KNbO3</strong><br />
BET (m 2 /g)<br />
Nb2O5 0.993 0.4476 1.2384 0.935 5.6180 0.5798 1.33<br />
<strong>KNbO3</strong> 0.914 1.4749 0.1799 0.912 1.6077 0.5428 4.02<br />
3µm<br />
3µm