Swissmedic Vigilance News Edition 21 – November 2018
In this edition: Isotretinoin and DOAC – Updates Confusion between amphotericin B formulations Guest articles: RPVC Zurich and RPVC Ticino Quality Assurance in Transfusion Practice Statistical Review 2017
In this edition:
Isotretinoin and DOAC – Updates
Confusion between amphotericin B formulations
Guest articles: RPVC Zurich and RPVC Ticino
Quality Assurance in Transfusion Practice
Statistical Review 2017
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
episode of hepatitis while taking the betalactam<br />
cefuroxime, the causality between<br />
the acute hepatitis and amoxicillin/clavulanic<br />
acid was formally rated as probable according<br />
to the CIOMS/WHO criteria.<br />
The following measures were recommended:<br />
the issuing of an allergy card for<br />
beta-lactams, the alternative use of other<br />
antibiotic groups with no structural similarity,<br />
such as macrolides or fluoroquinolones,<br />
or the administration of beta-lactams only in<br />
life-threatening situations.<br />
The Swiss RPVC Approach: Lessons for the<br />
future<br />
The Swiss spontaneous reporting system for<br />
postmarketing safety monitoring enables<br />
medical professionals to report ADR to pharmacovigilance<br />
centres. Such systems allow<br />
physicians and pharmacists to become directly<br />
involved in the safety monitoring system<br />
and can help them in their duty to report<br />
reactions as required by the Swiss Federal<br />
Act on Medicinal Products and Medical<br />
Devices. Detailed information on relevant<br />
symptoms, diagnostic results, history details,<br />
concomitant medication and the subsequent<br />
clinical outcome of the ADR can be reported<br />
using these systems. This detailed information<br />
directly from the healthcare professional<br />
is particularly important for ADR that<br />
are serious or rare, and is also an essential<br />
component of the postmarketing pharmacovigilance<br />
system. The remit is very broad<br />
and encompasses all medicines that are used<br />
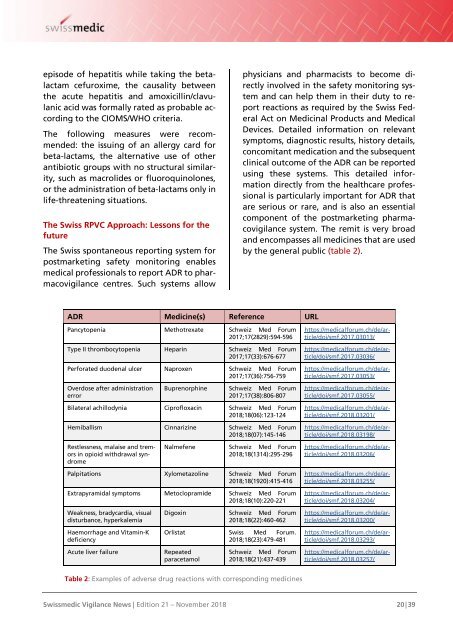
by the general public (table 2).<br />
ADR Medicine(s) Reference URL<br />
Pancytopenia Methotrexate Schweiz Med Forum<br />
2017;17(2829):594-596<br />
Type II thrombocytopenia Heparin Schweiz Med Forum<br />
2017;17(33):676-677<br />
Perforated duodenal ulcer Naproxen Schweiz Med Forum<br />
2017;17(36):756-759<br />
Overdose after administration<br />
error<br />
Buprenorphine Schweiz Med Forum<br />
2017;17(38):806-807<br />
Bilateral achillodynia Ciprofloxacin Schweiz Med Forum<br />
<strong>2018</strong>;18(06):123-124<br />
Hemiballism Cinnarizine Schweiz Med Forum<br />
<strong>2018</strong>;18(07):145-146<br />
https://medicalforum.ch/de/article/doi/smf.2017.03013/<br />
https://medicalforum.ch/de/article/doi/smf.2017.03036/<br />
https://medicalforum.ch/de/article/doi/smf.2017.03053/<br />
https://medicalforum.ch/de/article/doi/smf.2017.03055/<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03201/<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03198/<br />
Nalmefene Schweiz Med Forum<br />
<strong>2018</strong>;18(1314):295-296<br />
Palpitations Xylometazoline Schweiz Med Forum<br />
<strong>2018</strong>;18(1920):415-416<br />
Extrapyramidal symptoms Metoclopramide Schweiz Med Forum<br />
<strong>2018</strong>;18(10):220-2<strong>21</strong><br />
Weakness, bradycardia, visual<br />
disturbance, hyperkalemia<br />
Haemorrhage and Vitamin-K<br />
deficiency<br />
Digoxin Schweiz Med Forum<br />
<strong>2018</strong>;18(22):460-462<br />
Orlistat Swiss Med Forum.<br />
<strong>2018</strong>;18(23):479-481<br />
Restlessness, malaise and tremors<br />
in opioid withdrawal syndrome<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03206/<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03255/<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03204/<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03200/<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03293/<br />
Acute liver failure<br />
Repeated<br />
paracetamol<br />
Schweiz Med Forum<br />
<strong>2018</strong>;18(<strong>21</strong>):437-439<br />
https://medicalforum.ch/de/article/doi/smf.<strong>2018</strong>.03257/<br />
Table 2: Examples of adverse drug reactions with corresponding medicines<br />
<strong>Swissmedic</strong> <strong>Vigilance</strong> <strong>News</strong> | <strong>Edition</strong> <strong>21</strong> <strong>–</strong> <strong>November</strong> <strong>2018</strong> 20 | 39