Self-Assembled Nanoreactors - Cluster for Molecular Chemistry
Self-Assembled Nanoreactors - Cluster for Molecular Chemistry
Self-Assembled Nanoreactors - Cluster for Molecular Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1460 Chemical Reviews, 2005, Vol. 105, No. 4 Vriezema et al.<br />
Chart 3<br />
and with the uncatalyzed reaction in acetonitrile. It<br />
was found that the rate increased by 1.5-2-fold when<br />
going from the micellar to the vesicular system, but<br />
the rate enhancement as compared to the uncatalyzed<br />
reaction was million fold. Furthermore, it was<br />
observed that catalysis already occurred at 10-20<br />
times lower concentration <strong>for</strong> the metallo-vesicles<br />
than <strong>for</strong> the metallo-micelles. More efficient binding<br />
of the substrate to the small vesicles possibly explains<br />
the observed differences. 161 In these examples, the<br />
counterion is the active catalyst. In the following part<br />
of this section, pre<strong>for</strong>med (transition) metal complexes<br />
anchored to vesicular nanoreactors act in this<br />
way.<br />
Cytochrome P450 is an oxidative enzyme involved<br />
in a diversity of natural processes; 162,163 next to<br />
oxidations it is also active in dehydrogenation reactions,<br />
oxidative <strong>for</strong>mylations, dehydrations, etc. 164<br />
Cytochrome P450 is a membrane-bound enzyme, a<br />
feature which prompted numerous research groups<br />
to design model systems in which metal porphyrins<br />
(a synthetic equivalent of the iron(III) protoporphyrin<br />
IX present in the active center of the enzyme) are<br />
incorporated in the bilayers of vesicle membranes. 165<br />
The first studies in this direction were conducted by<br />
Sorokin et al. in the early 1980s. 166 Oxidation reactions<br />
catalyzed by the relatively simple complex<br />
manganese(III) tetrahexadecylphenyl porphyrin chloride<br />
incorporated in the membrane of DMPC (Table<br />
1) vesicles were studied using iodosobenzene as an<br />
oxidant. In a comparable system built from DMPC<br />
or DPPC (Table 1) vesicles, regioselectivity was<br />
obtained in the epoxidation of steroids and polyunsaturated<br />
fatty acids by employing a membrane<br />
spanning iron(III) tetrakis(o-cholenylamidophenyl)porphyrin<br />
(Fe(III)ChPP) as catalyst. 167 The same<br />
porphyrin, with a different metal in its central core<br />
(i.e., Mn(III) ChPP), was used to construct a selfassembled<br />
system, which by reductive activation of<br />
molecular oxygen produced 20 mol of acetophenone<br />
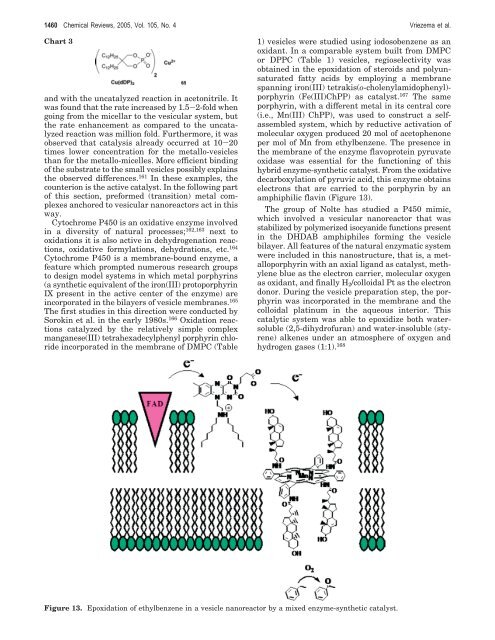
per mol of Mn from ethylbenzene. The presence in<br />
the membrane of the enzyme flavoprotein pyruvate<br />
oxidase was essential <strong>for</strong> the functioning of this<br />
hybrid enzyme-synthetic catalyst. From the oxidative<br />
decarboxylation of pyruvic acid, this enzyme obtains<br />
electrons that are carried to the porphyrin by an<br />
amphiphilic flavin (Figure 13).<br />
The group of Nolte has studied a P450 mimic,<br />
which involved a vesicular nanoreactor that was<br />
stabilized by polymerized isocyanide functions present<br />
in the DHDAB amphiphiles <strong>for</strong>ming the vesicle<br />
bilayer. All features of the natural enzymatic system<br />
were included in this nanostructure, that is, a metalloporphyrin<br />
with an axial ligand as catalyst, methylene<br />
blue as the electron carrier, molecular oxygen<br />
as oxidant, and finally H2/colloidal Pt as the electron<br />
donor. During the vesicle preparation step, the porphyrin<br />
was incorporated in the membrane and the<br />
colloidal platinum in the aqueous interior. This<br />
catalytic system was able to epoxidize both watersoluble<br />
(2,5-dihydrofuran) and water-insoluble (styrene)<br />
alkenes under an atmosphere of oxygen and<br />
hydrogen gases (1:1). 168<br />
Figure 13. Epoxidation of ethylbenzene in a vesicle nanoreactor by a mixed enzyme-synthetic catalyst.