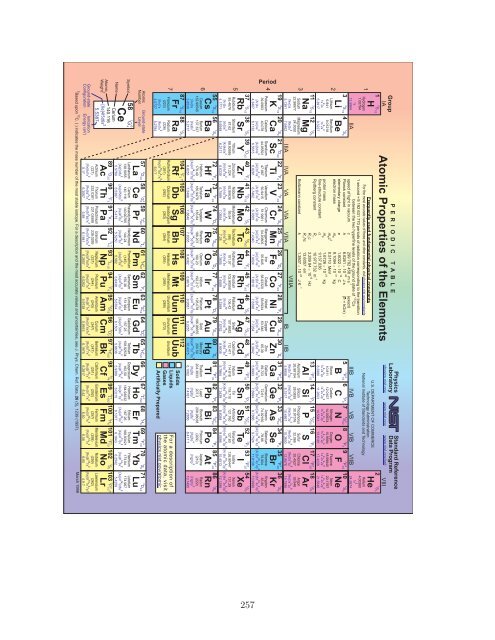
Quantum Mechanics - Prof. Eric R. Bittner - University of Houston
Quantum Mechanics - Prof. Eric R. Bittner - University of Houston
Quantum Mechanics - Prof. Eric R. Bittner - University of Houston
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Ground-state<br />
Configuration<br />
Protactinium<br />
Neptunium Plutonium Americium<br />
Berkelium Californium Einsteinium Fermium Mendelevium<br />
(227) 232.0381 231.03588 238.0289 (237) (244) (243) (247) (247) (251) (252) (257) (258)<br />
[Rn]6d7s<br />
5.17 6.3067 5.89 6.1941 6.2657 6.0262 5.9738 5.9915 6.1979 6.2817 6.42 6.50 6.58<br />
2<br />
Ionization<br />
2 2<br />
2 2<br />
3 2<br />
4 2<br />
6 2<br />
7 2<br />
7 2<br />
9 2<br />
10 2<br />
11 2<br />
12 2<br />
13 2<br />
[Rn]6d 7s [Rn]5f 6d7s [Rn]5f 6d7s [Rn]5f 6d7s [Rn]5f 7s [Rn]5f 7s [Rn]5f 6d7s [Rn]5f 7s [Rn]5f 7s [Rn]5f 7s [Rn]5f 7s [Rn]5f 7s<br />
Energy (eV)<br />
† 12<br />
Based upon C.<br />
( ) indicates the mass number <strong>of</strong> the most stable isotope. For a description and the most accurate values and uncertainties, see J. Phys. Chem. Ref. Data, 26 (5), 1239 (1997).<br />
March 1999<br />
Nobelium Lawrencium<br />
(259) (262)<br />
14 2 14 2<br />
[Rn]5f 7s [Rn]5f 7s 7p?<br />
6.65 4.9 ?<br />
Atomic<br />
Weight †<br />
140.116<br />
[Xe]4f5d6s<br />
5.5387<br />
2<br />
89<br />
Ac<br />
Actinium<br />
2<br />
D32 /<br />
90<br />
Th<br />
Thorium<br />
91<br />
Pa<br />
4<br />
K11/ 2<br />
92<br />
U<br />
Uranium<br />
103<br />
Lr<br />
Lanthanum<br />
138.9055<br />
[Xe]5d6s<br />
5.5769<br />
2<br />
Praseodymium Neodymium Promethium<br />
140.116 140.90765 144.24 (145)<br />
[Xe]4f5d6s<br />
5.5387 5.473 5.5250 5.582<br />
2<br />
3 2<br />
4 2<br />
5 2<br />
[Xe]4f 6s [Xe]4f 6s [Xe]4f 6s<br />
3 5<br />
F2<br />
L°6<br />
93<br />
Np<br />
6<br />
L11/ 2<br />
94<br />
Pu<br />
7 F0<br />
95<br />
Am<br />
8<br />
S°7<br />
/ 2<br />
96<br />
Cm<br />
Curium<br />
9 D°2<br />
97<br />
Bk<br />
6<br />
H°15<br />
/ 2<br />
98<br />
Cf<br />
5- I-8<br />
99<br />
Es<br />
4-<br />
I-° 15/ 2<br />
100<br />
Fm<br />
3 H6<br />
101<br />
Md<br />
2<br />
F°7<br />
/ 2<br />
102<br />
No<br />
1 S0<br />
2<br />
P°1<br />
/ 2 ?<br />
Name<br />
Symbol<br />
Samarium<br />
150.36<br />
6 2<br />
[Xe]4f 6s<br />
5.6436<br />
Europium<br />
151.964<br />
7 2<br />
[Xe]4f 6s<br />
5.6704<br />
Gadolinium<br />
157.25<br />
7 2<br />
[Xe]4f 5d6s<br />
6.1501<br />
158.92534<br />
9 2<br />
[Xe]4f 6s<br />
5.8638<br />
Dysprosium<br />
162.50<br />
10 2<br />
[Xe]4f 6s<br />
5.9389<br />
Holmium<br />
164.93032<br />
11 2<br />
[Xe]4f 6s<br />
6.0215<br />
167.26<br />
12 2<br />
[Xe]4f 6s<br />
6.1077<br />
168.93421<br />
13 2<br />
[Xe]4f 6s<br />
6.1843<br />
Ytterbium<br />
173.04<br />
14 2<br />
[Xe]4f 6s<br />
6.2542<br />
174.967<br />
14 2<br />
[Xe]4f 5d6s<br />
5.4259<br />
58<br />
Ce<br />
Cerium<br />
1 G°4<br />
Atomic<br />
Number<br />
Ground-state<br />
Level<br />
57<br />
La<br />
2<br />
D32 /<br />
58<br />
Ce<br />
Cerium<br />
1 G°4<br />
59<br />
Pr<br />
4-<br />
I-° 92 /<br />
60<br />
Nd<br />
5- I-4<br />
61<br />
Pm<br />
6<br />
H°5<br />
/ 2<br />
62<br />
Sm<br />
7 F0<br />
63<br />
Eu<br />
8<br />
S°7<br />
/ 2<br />
64<br />
Gd<br />
9 D°2<br />
65<br />
Tb<br />
Terbium<br />
6<br />
H°15<br />
/ 2<br />
66<br />
Dy<br />
5- I-8<br />
67<br />
Ho<br />
4-<br />
I-° 15/ 2<br />
68<br />
Er<br />
Erbium<br />
3 H6<br />
69<br />
Tm<br />
Thulium<br />
2<br />
F°7<br />
/ 2<br />
70<br />
Yb<br />
1 S0<br />
71<br />
Lu<br />
Lutetium<br />
2<br />
D32 /<br />
Francium<br />
(223)<br />
[Rn]7s<br />
4.0727<br />
(226)<br />
[Rn]7s<br />
5.2784<br />
2<br />
7<br />
Rutherfordium Dubnium<br />
(261) (262)<br />
14 2 2<br />
[Rn]5f 6d 7s ?<br />
6.0 ?<br />
87<br />
Fr<br />
88<br />
Ra<br />
Radium<br />
Seaborgium<br />
(263)<br />
104<br />
Rf<br />
Db<br />
Sg<br />
Bh<br />
Bohrium<br />
(264)<br />
Hassium<br />
(265)<br />
Meitnerium<br />
(268)<br />
Ununnilium<br />
(269)<br />
Unununium<br />
(272)<br />
Ununbium<br />
For a description <strong>of</strong><br />
the atomic data, visit<br />
physics.nist.gov/atomic<br />
2<br />
S12 /<br />
1 S0<br />
Hs<br />
Mt<br />
Uun<br />
Uuu<br />
Uub<br />
Solids<br />
Liquids<br />
Gases<br />
Artificially Prepared<br />
132.90545<br />
[Xe]6s<br />
3.8939<br />
137.327<br />
[Xe]6s<br />
5.2117<br />
2<br />
Rhenium<br />
178.49 180.9479 183.84 186.207 190.23 192.217 195.078 196.96655 200.59 204.3833 207.2<br />
14 2 2 14 3 2 14 4 2 14 5 2 14 6 2 14 7 2 14 9<br />
14 10<br />
14 10 2<br />
[Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Xe]4f 5d 6s [Hg]6p [Hg]6p<br />
6.8251 7.5496 7.8640 7.8335 8.4382 8.9670 8.9587 9.2255 10.4375 6.1082 7.4167<br />
2<br />
3<br />
F2 ? 105 106 107 108 109 110 111 112<br />
208.98038<br />
[Hg]6p<br />
7.2856<br />
3<br />
Polonium<br />
(209)<br />
[Hg]6p<br />
8.417 ?<br />
4<br />
[Hg]6p 5<br />
(210)<br />
(222)<br />
[Hg]6p<br />
10.7485<br />
6<br />
6<br />
55<br />
Cs<br />
Cesium<br />
2<br />
S12 /<br />
56<br />
Ba<br />
Barium<br />
1 S0<br />
72<br />
Hf<br />
Hafnium<br />
3 F2<br />
73<br />
Ta<br />
Tantalum<br />
4<br />
F32 /<br />
74<br />
W<br />
Tungsten<br />
5 D0<br />
75<br />
Re<br />
6<br />
S52 /<br />
76<br />
Os<br />
Osmium<br />
5 D4<br />
77<br />
Ir<br />
Iridium<br />
4<br />
F92 /<br />
78<br />
Pt<br />
Platinum<br />
3 D3<br />
79<br />
Au<br />
Gold<br />
2<br />
S12 /<br />
80<br />
Hg<br />
Mercury<br />
81<br />
Tl<br />
Thallium<br />
2<br />
P°1/ 2<br />
Rubidium<br />
85.4678<br />
[Kr]5s<br />
4.1771<br />
87.62<br />
[Kr]5s<br />
5.6949<br />
2<br />
88.90585<br />
[Kr]4d5s<br />
6.2171<br />
2<br />
91.224<br />
2 2<br />
[Kr]4d 5s<br />
6.6339<br />
6.7589<br />
92.90638<br />
Molybdenum Technetium<br />
95.94 (98)<br />
5<br />
5 2<br />
[Kr]4d 5s [Kr]4d 5s<br />
7.0924 7.28<br />
Cadmium<br />
Antimony<br />
112.411 114.818 118.710 121.760 127.60 126.90447 131.29<br />
10 2<br />
10 2<br />
10 2 2 10 2 3 10 2 4 10 2 5 10 2 6<br />
[Kr]4d 5s [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p [Kr]4d 5s 5p<br />
8.9938 5.7864 7.3439 8.6084 9.0096 10.4513 12.1298<br />
1 3 3 1<br />
S0<br />
P0<br />
P2<br />
S0<br />
82<br />
Pb<br />
Lead<br />
83<br />
Bi<br />
Bismuth<br />
4<br />
S°3/ 2<br />
84<br />
Po<br />
85<br />
At<br />
Astatine<br />
2<br />
P°3/ 2<br />
86<br />
Rn<br />
Radon<br />
Ruthenium<br />
101.07<br />
7<br />
[Kr]4d 5s<br />
7.3605<br />
Rhodium<br />
102.90550<br />
8<br />
[Kr]4d 5s<br />
7.4589<br />
Palladium<br />
106.42<br />
[Kr]4d<br />
8.3369<br />
10<br />
7.5762<br />
4<br />
[Kr]4d 5s<br />
10<br />
[Kr]4d 5s<br />
107.8682<br />
5<br />
37<br />
Rb<br />
2<br />
S12 /<br />
38<br />
Sr<br />
Strontium<br />
1 S0<br />
39<br />
Y<br />
Yttrium<br />
2<br />
D32 /<br />
40<br />
Zr<br />
Zirconium<br />
3 F2<br />
41<br />
Nb<br />
Niobium<br />
6<br />
D12 /<br />
42<br />
Mo<br />
7 S3<br />
43<br />
Tc<br />
6<br />
S52 /<br />
44<br />
Ru<br />
5 F5<br />
45<br />
Rh<br />
4<br />
F92 /<br />
46<br />
Pd<br />
1 S0<br />
47<br />
Ag<br />
Silver<br />
2<br />
S12 /<br />
48<br />
Cd<br />
49<br />
In<br />
Indium<br />
2<br />
P°1/ 2<br />
54<br />
Xe<br />
Xenon<br />
Potassium<br />
39.0983<br />
[Ar]4s<br />
4.3407<br />
40.078<br />
[Ar]4s<br />
6.1132<br />
2<br />
Scandium<br />
44.95591<br />
[Ar]3d4s<br />
6.5615<br />
2<br />
47.867<br />
2 2<br />
[Ar]3d 4s<br />
6.8281<br />
Vanadium<br />
50.9415<br />
3 2<br />
[Ar]3d 4s<br />
6.7462<br />
6.7665<br />
51.9961<br />
Manganese<br />
54.93805<br />
5 2<br />
[Ar]3d 4s<br />
7.4340<br />
55.845<br />
6 2<br />
[Ar]3d 4s<br />
7.9024<br />
58.93320<br />
7 2<br />
[Ar]3d 4s<br />
7.8810<br />
58.6934<br />
8 2<br />
[Ar]3d 4s<br />
7.6398<br />
7.7264<br />
63.546<br />
Germanium<br />
65.39 69.723 72.61 74.92160 78.96 79.904 83.80<br />
10 2<br />
10 2<br />
10 2 2 10 2 3 10 2 4 10 2 5 10 2 6<br />
[Ar]3d 4s [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p [Ar]3d 4s 4p<br />
9.3942 5.9993 7.8994 9.7886 9.7524 11.8138 13.9996<br />
1 3 3 1<br />
S0<br />
P0<br />
P2<br />
S0<br />
50<br />
Sn<br />
Tin<br />
51<br />
Sb<br />
4<br />
S°3/ 2<br />
52<br />
Te<br />
Tellurium<br />
53<br />
I<br />
Iodine<br />
2<br />
P°3/ 2<br />
257<br />
Period<br />
5<br />
[Ar]3d 4s<br />
10<br />
[Ar]3d 4s<br />
4<br />
19<br />
K<br />
2<br />
S12 /<br />
20<br />
Ca<br />
Calcium<br />
1 S0<br />
21<br />
Sc<br />
22<br />
Ti<br />
Titanium<br />
23<br />
V<br />
24<br />
Cr<br />
Chromium<br />
Mn<br />
26<br />
Fe<br />
Iron<br />
30<br />
Zn<br />
Zinc<br />
31<br />
Ga<br />
Gallium<br />
32<br />
Ge<br />
33<br />
As<br />
Arsenic<br />
34<br />
Se<br />
Selenium<br />
35<br />
Br<br />
Bromine<br />
36<br />
Kr<br />
Krypton<br />
IIIA IVA VA VIA VIIA<br />
3 7<br />
F2<br />
S3 25<br />
2<br />
D32 /<br />
4<br />
F32 /<br />
6<br />
S52 /<br />
5 D4<br />
27<br />
Co<br />
Cobalt<br />
4<br />
F92 /<br />
28<br />
Ni<br />
Nickel<br />
3 F4<br />
Cu<br />
Copper<br />
22.98977<br />
[Ne]3s<br />
5.1391<br />
Magnesium<br />
24.3050<br />
[Ne]3s<br />
7.6462<br />
2<br />
VIIIA<br />
IB IIB<br />
29<br />
2<br />
S12 /<br />
1 S0<br />
2<br />
P°1/ 2<br />
3 P0<br />
4<br />
S°3/ 2<br />
3 P2<br />
2<br />
P°3/ 2<br />
1 S0<br />
Aluminum<br />
26.98154<br />
2<br />
[Ne]3s 3p<br />
5.9858<br />
28.0855<br />
2 2<br />
[Ne]3s 3p<br />
8.1517<br />
Phosphorus<br />
30.97376<br />
2 3<br />
[Ne]3s 3p<br />
10.4867<br />
32.066<br />
2 4<br />
[Ne]3s 3p<br />
10.3600<br />
35.4527<br />
2 5<br />
[Ne]3s 3p<br />
12.9676<br />
39.948<br />
2 6<br />
[Ne]3s 3p<br />
15.7596<br />
3<br />
11<br />
Na<br />
Sodium<br />
2<br />
S12 /<br />
12<br />
Mg<br />
1 S0<br />
13<br />
Al<br />
14<br />
Si<br />
Silicon<br />
15<br />
P<br />
16<br />
S<br />
Sulfur<br />
17<br />
Cl<br />
Chlorine<br />
18<br />
Ar<br />
Argon<br />
5.3917<br />
2<br />
1s 2s<br />
6.941<br />
Beryllium<br />
9.01218<br />
2 2<br />
1s 2s<br />
9.3227<br />
10.811<br />
2 2<br />
1s 2s 2p<br />
8.2980<br />
12.0107<br />
2 2 2<br />
1s 2s 2p<br />
11.2603<br />
14.00674<br />
2 2 3<br />
1s 2s 2p<br />
14.5341<br />
15.9994<br />
2 2 4<br />
1s 2s 2p<br />
13.6181<br />
18.99840<br />
2 2 5<br />
1s 2s 2p<br />
17.4228<br />
20.1797<br />
2 2 6<br />
1s 2s 2p<br />
21.5646<br />
2<br />
Li<br />
Lithium<br />
B<br />
Boron<br />
C<br />
Carbon<br />
N<br />
Nitrogen<br />
O<br />
Oxygen<br />
F<br />
Fluorine<br />
3<br />
2<br />
S12 /<br />
4<br />
Be<br />
10<br />
Ne<br />
Neon<br />
Hydrogen<br />
1.00794<br />
1s<br />
13.5984<br />
IIA<br />
IIIB IVB VB VIB VIIB<br />
3 3<br />
5 6 P0 7 8 P2 9<br />
1s 2<br />
4.00260<br />
24.5874<br />
1<br />
H<br />
Frequently used fundamental physical constants<br />
For the most accurate values <strong>of</strong> these and other constants, visit physics.nist.gov/constants<br />
1 second = 9 192 631 770 periods <strong>of</strong> radiation corresponding to the transition<br />
133<br />
between the two hyperfine levels <strong>of</strong> the ground state <strong>of</strong> Cs<br />
�1<br />
speed <strong>of</strong> light in vacuum c 299 792 458 m s (exact)<br />
�34<br />
Planck constant h 6.6261 � 10 J s ( h=h/2<br />
�)<br />
�19<br />
elementary charge e 1.6022 � 10 C<br />
�31<br />
electron mass me<br />
9.1094 � 10 kg<br />
2<br />
mc e 0.5110 MeV<br />
�27<br />
proton mass mp<br />
1.6726 � 10 kg<br />
fine-structure constant � 1/137.036<br />
�1<br />
Rydberg constant R�<br />
10 973 732 m<br />
15<br />
Rc � 3.289 84 � 10 Hz<br />
Rhc � 13.6057 eV<br />
�23 �1<br />
Boltzmann constant k 1.3807 � 10 J K<br />
2<br />
P°1/ 2<br />
3 P0<br />
4<br />
S°3/ 2<br />
3 P2<br />
2<br />
P°3/ 2<br />
1 S0<br />
1 S0<br />
2<br />
P°1<br />
/ 2<br />
4<br />
S°3<br />
/ 2<br />
2<br />
P°3<br />
/ 2<br />
1 S0<br />
U.S. DEPARTMENT OF COMMERCE<br />
Technology Administration<br />
National Institute <strong>of</strong> Standards and Technology<br />
He<br />
Helium<br />
Group<br />
IA<br />
1<br />
2<br />
S12 /<br />
P E R I O D I C T A B L E<br />
Atomic Properties <strong>of</strong> the Elements<br />
physics.nist.gov www.nist.gov www.nist.gov/srd<br />
Physics<br />
Laboratory<br />
Standard Reference<br />
Data Program<br />
VIII<br />
2<br />
1 S0