A Facile and Green Synthesis of Sulforaphane
A Facile and Green Synthesis of Sulforaphane
A Facile and Green Synthesis of Sulforaphane
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
HO HO<br />
HO<br />
HO<br />
Tong Jian DING et al. 1153<br />
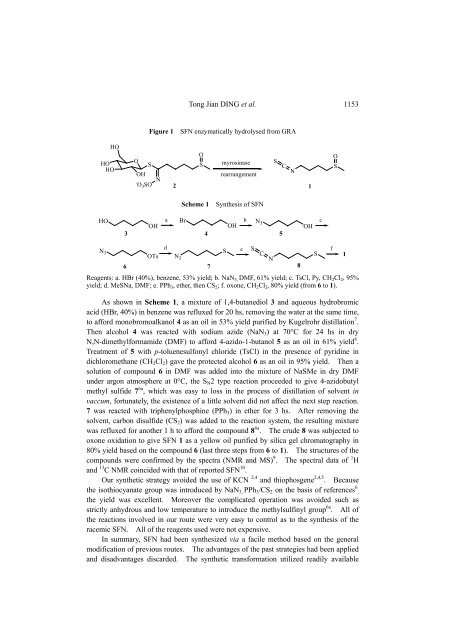
Figure 1 SFN enzymatically hydrolysed from GRA<br />
O<br />
OH<br />
-O3SO 2<br />
myrosinase<br />
rearrangement<br />
1<br />
N<br />
S<br />
O<br />
S<br />
S<br />
C<br />
N<br />
Scheme 1 <strong>Synthesis</strong> <strong>of</strong> SFN<br />
OH<br />
a Br<br />
OH<br />
b N3 3 4 5<br />
N3 OTs<br />
d<br />
N3 S e S<br />
C<br />
N<br />
S<br />
f<br />
1<br />
6 7 8<br />
Reagents: a. HBr (40%), benzene, 53% yield; b. NaN3, DMF, 61% yield; c. TsCl, Py, CH2Cl2, 95%<br />
yield; d. MeSNa, DMF; e. PPh3, ether, then CS2; f. oxone, CH2Cl2, 80% yield (from 6 to 1).<br />
As shown in Scheme 1, a mixture <strong>of</strong> 1,4-butanediol 3 <strong>and</strong> aqueous hydrobromic<br />
acid (HBr, 40%) in benzene was refluxed for 20 hs, removing the water at the same time,<br />
to afford monobromoalkanol 4 as an oil in 53% yield purified by Kugelrohr distillation 7 .<br />
Then alcohol 4 was reacted with sodium azide (NaN3) at 70°C for 24 hs in dry<br />
N,N-dimethylformamide (DMF) to afford 4-azido-1-butanol 5 as an oil in 61% yield 8 .<br />
Treatment <strong>of</strong> 5 with p-toluenesulfonyl chloride (TsCl) in the presence <strong>of</strong> pyridine in<br />
dichloromethane (CH2Cl2) gave the protected alcohol 6 as an oil in 95% yield. Then a<br />
solution <strong>of</strong> compound 6 in DMF was added into the mixture <strong>of</strong> NaSMe in dry DMF<br />
under argon atmosphere at 0°C, the SN2 type reaction proceeded to give 4-azidobutyl<br />
methyl sulfide 7 6a , which was easy to loss in the process <strong>of</strong> distillation <strong>of</strong> solvent in<br />
vaccum, fortunately, the existence <strong>of</strong> a little solvent did not affect the next step reaction.<br />
7 was reacted with triphenylphosphine (PPh3) in ether for 3 hs. After removing the<br />
solvent, carbon disulfide (CS2) was added to the reaction system, the resulting mixture<br />
was refluxed for another 1 h to afford the compound 8 6a . The crude 8 was subjected to<br />
oxone oxidation to give SFN 1 as a yellow oil purified by silica gel chromatography in<br />
80% yield based on the compound 6 (last three steps from 6 to 1). The structures <strong>of</strong> the<br />
compounds were confirmed by the spectra (NMR <strong>and</strong> MS) 9 . The spectral data <strong>of</strong> 1 H<br />
<strong>and</strong> 13 C NMR coincided with that <strong>of</strong> reported SFN 10 .<br />
Our synthetic strategy avoided the use <strong>of</strong> KCN 2,4 <strong>and</strong> thiophosgene 2,4,5 . Because<br />
the isothiocyanate group was introduced by NaN3, PPh3/CS2 on the basis <strong>of</strong> references 6 ,<br />
the yield was excellent. Moreover the complicated operation was avoided such as<br />
strictly anhydrous <strong>and</strong> low temperature to introduce the methylsulfinyl group 6a . All <strong>of</strong><br />
the reactions involved in our route were very easy to control as to the synthesis <strong>of</strong> the<br />
racemic SFN. All <strong>of</strong> the reagents used were not expensive.<br />
In summary, SFN had been synthesized via a facile method based on the general<br />
modification <strong>of</strong> previous routes. The advantages <strong>of</strong> the past strategies had been applied<br />
<strong>and</strong> disadvantages discarded. The synthetic transformation utilized readily available<br />
OH<br />
c<br />
O<br />
S