Temperature (1) - Oxford University Press
Temperature (1) - Oxford University Press
Temperature (1) - Oxford University Press
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
102<br />
THERMAL T<br />
EFFECTS<br />
ice<br />
point<br />
0 °C<br />
pure<br />
melting<br />
ice<br />
5.03<br />
� Finding the lower fixed point<br />
steam<br />
point<br />
100 °C<br />
boiling<br />
water<br />
� Finding the upper fixed point<br />
<strong>Temperature</strong> (2)<br />
Fixing a temperature scale<br />
To create a temperature scale, two standard temperatures must be chosen<br />
against which others can be judged. These fixed points need to be defined so<br />
that they can be reproduced in laboratories anywhere in the world:<br />
On the Celsius scale:<br />
0 degrees Celsius (0 �C) is defined as the melting point of pure ice. This is<br />
the lower fixed point, known as the ice point.<br />
100 degrees Celsius (100 �C) is defined as the boiling point of pure water,<br />
where the water is boiling under standard atmospheric pressure (101 325 Pa).<br />
This is the upper fixed point, known as the steam point.<br />
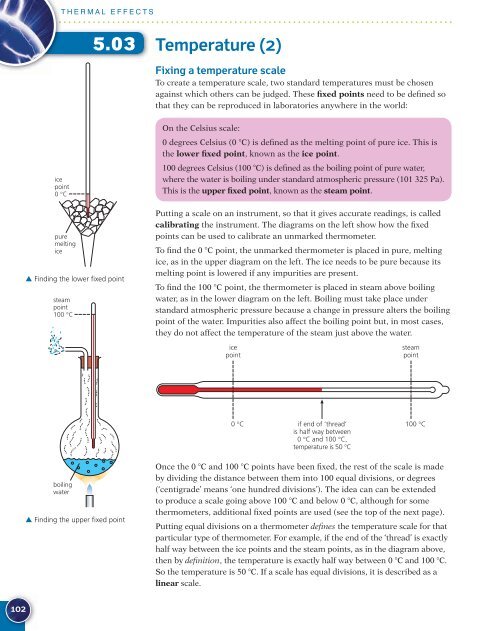
Putting a scale on an instrument, so that it gives accurate readings, is called<br />
calibrating the instrument. The diagrams on the left show how the fixed<br />
points can be used to calibrate an unmarked thermometer.<br />
To find the 0 �C point, the unmarked thermometer is placed in pure, melting<br />
ice, as in the upper diagram on the left. The ice needs to be pure because its<br />
melting point is lowered if any impurities are present.<br />
To find the 100 �C point, the thermometer is placed in steam above boiling<br />
water, as in the lower diagram on the left. Boiling must take place under<br />
standard atmospheric pressure because a change in pressure alters the boiling<br />
point of the water. Impurities also affect the boiling point but, in most cases,<br />
they do not affect the temperature of the steam just above the water.<br />
ice<br />
point<br />
steam<br />
point<br />
0 °C if end of ’thread‘<br />
is half way between<br />
0 °C and 100 °C,<br />
temperature is 50 °C<br />
100 °C<br />
Once the 0 �C and 100 �C points have been fixed, the rest of the scale is made<br />
by dividing the distance between them into 100 equal divisions, or degrees<br />
(‘centigrade’ means ‘one hundred divisions’). The idea can can be extended<br />
to produce a scale going above 100 �C and below 0 �C, although for some<br />
thermometers, additional fixed points are used (see the top of the next page).<br />
Putting equal divisions on a thermometer defines the temperature scale for that<br />
particular type of thermometer. For example, if the end of the ‘thread’ is exactly<br />
half way between the ice points and the steam points, as in the diagram above,<br />
then by definition, the temperature is exactly half way between 0 �C and 100 �C.<br />
So the temperature is 50 �C. If a scale has equal divisions, it is described as a<br />
linear scale.