Endocrine control of ovarian function in dogs and other carnivores
Endocrine control of ovarian function in dogs and other carnivores
Endocrine control of ovarian function in dogs and other carnivores
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Concannon et al. Ovarian <strong>function</strong> <strong>in</strong> <strong>dogs</strong> <strong>and</strong> <strong>other</strong> <strong>carnivores</strong>.<br />
LH surge (Fig. 9). Therefore, <strong>in</strong> <strong>dogs</strong>, the late proestrus<br />
conditions caus<strong>in</strong>g the preovulatory surge release <strong>of</strong> LH<br />
likely <strong>in</strong>clude pituitary <strong>and</strong> hypothalamic effects <strong>of</strong><br />
these <strong>in</strong>creases <strong>in</strong> both progesterone <strong>and</strong> 17α-OH<br />
progesterone act<strong>in</strong>g via PMRs, both <strong>in</strong> trigger<strong>in</strong>g <strong>and</strong><br />
susta<strong>in</strong><strong>in</strong>g the LH surge. The cont<strong>in</strong>ued slow rise <strong>in</strong> both<br />
moieties just before surge onset would participate <strong>in</strong><br />
facilitat<strong>in</strong>g the surge-trigger<strong>in</strong>g action <strong>of</strong> the decl<strong>in</strong>e <strong>in</strong><br />
E:P primarily effected by peak<strong>in</strong>g estradiol. The late<br />
proestrus <strong>in</strong>creases <strong>in</strong> 17α likely supplement the<br />
facilitat<strong>in</strong>g effect <strong>of</strong> the simultaneous <strong>in</strong>creases rise <strong>in</strong><br />
LH (ng/ml)<br />
1000<br />
100<br />
10<br />
1<br />
0.1<br />
GnRH-A<br />
N=5<br />
Control<br />
N=4<br />
progesterone on the tim<strong>in</strong>g <strong>and</strong> magnitude <strong>of</strong> the LH<br />
surge.<br />
In the above view <strong>of</strong> the regulation <strong>of</strong> LH<br />
surge release as well as estrus behavior <strong>in</strong>volv<strong>in</strong>g a<br />
prim<strong>in</strong>g or potentiat<strong>in</strong>g by ris<strong>in</strong>g estradiol, <strong>and</strong><br />
trigger<strong>in</strong>g by a decl<strong>in</strong>e <strong>in</strong> the E:P ratio, the target cells<br />
are viewed as be<strong>in</strong>g responsive not simply to estrogen<br />
concentrations or any particular threshold estrogen<br />
concentrations, but to rather the rate <strong>of</strong> change (first<br />
derivative) dE/dt , <strong>and</strong> the rate <strong>of</strong> change <strong>in</strong> E:P ration,<br />
dE:P/dt (Fig. 8 <strong>and</strong> 10a, b).<br />
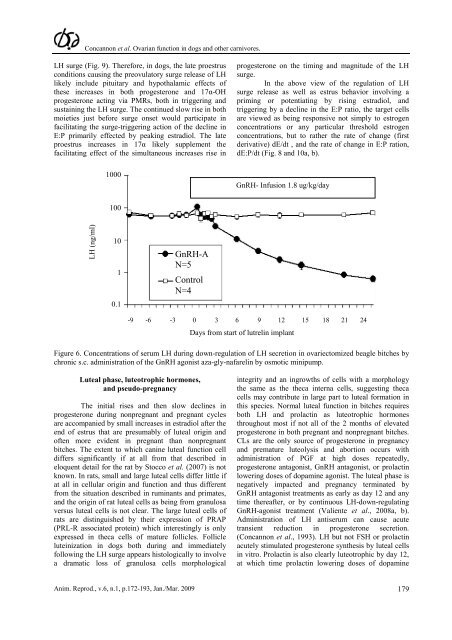
Figure 6. Concentrations <strong>of</strong> serum LH dur<strong>in</strong>g down-regulation <strong>of</strong> LH secretion <strong>in</strong> ovariectomized beagle bitches by<br />
chronic s.c. adm<strong>in</strong>istration <strong>of</strong> the GnRH agonist aza-gly-nafarel<strong>in</strong> by osmotic m<strong>in</strong>ipump.<br />
Luteal phase, luteotrophic hormones,<br />
<strong>and</strong> pseudo-pregnancy<br />
The <strong>in</strong>itial rises <strong>and</strong> then slow decl<strong>in</strong>es <strong>in</strong><br />
progesterone dur<strong>in</strong>g nonpregnant <strong>and</strong> pregnant cycles<br />
are accompanied by small <strong>in</strong>creases <strong>in</strong> estradiol after the<br />
end <strong>of</strong> estrus that are presumably <strong>of</strong> luteal orig<strong>in</strong> <strong>and</strong><br />
<strong>of</strong>ten more evident <strong>in</strong> pregnant than nonpregnant<br />
bitches. The extent to which can<strong>in</strong>e luteal <strong>function</strong> cell<br />
differs significantly if at all from that described <strong>in</strong><br />
eloquent detail for the rat by Stocco et al. (2007) is not<br />
known. In rats, small <strong>and</strong> large luteal cells differ little if<br />
at all <strong>in</strong> cellular orig<strong>in</strong> <strong>and</strong> <strong>function</strong> <strong>and</strong> thus different<br />
from the situation described <strong>in</strong> rum<strong>in</strong>ants <strong>and</strong> primates,<br />
<strong>and</strong> the orig<strong>in</strong> <strong>of</strong> rat luteal cells as be<strong>in</strong>g from granulosa<br />
versus luteal cells is not clear. The large luteal cells <strong>of</strong><br />
rats are dist<strong>in</strong>guished by their expression <strong>of</strong> PRAP<br />
(PRL-R associated prote<strong>in</strong>) which <strong>in</strong>terest<strong>in</strong>gly is only<br />
expressed <strong>in</strong> theca cells <strong>of</strong> mature follicles. Follicle<br />
lute<strong>in</strong>ization <strong>in</strong> <strong>dogs</strong> both dur<strong>in</strong>g <strong>and</strong> immediately<br />
follow<strong>in</strong>g the LH surge appears histologically to <strong>in</strong>volve<br />
a dramatic loss <strong>of</strong> granulosa cells morphological<br />
GnRH- Infusion 1.8 ug/kg/day<br />
-9 -6 -3 0 3 6 9 12 15 18 21 24<br />
Days from start <strong>of</strong> lutrel<strong>in</strong> implant<br />
<strong>in</strong>tegrity <strong>and</strong> an <strong>in</strong>growths <strong>of</strong> cells with a morphology<br />
the same as the theca <strong>in</strong>terna cells, suggest<strong>in</strong>g theca<br />
cells may contribute <strong>in</strong> large part to luteal formation <strong>in</strong><br />
this species. Normal luteal <strong>function</strong> <strong>in</strong> bitches requires<br />
both LH <strong>and</strong> prolact<strong>in</strong> as luteotrophic hormones<br />
throughout most if not all <strong>of</strong> the 2 months <strong>of</strong> elevated<br />
progesterone <strong>in</strong> both pregnant <strong>and</strong> nonpregnant bitches.<br />
CLs are the only source <strong>of</strong> progesterone <strong>in</strong> pregnancy<br />
<strong>and</strong> premature luteolysis <strong>and</strong> abortion occurs with<br />
adm<strong>in</strong>istration <strong>of</strong> PGF at high doses repeatedly,<br />
progesterone antagonist, GnRH antagonist, or prolact<strong>in</strong><br />
lower<strong>in</strong>g doses <strong>of</strong> dopam<strong>in</strong>e agonist. The luteal phase is<br />
negatively impacted <strong>and</strong> pregnancy term<strong>in</strong>ated by<br />
GnRH antagonist treatments as early as day 12 <strong>and</strong> any<br />
time thereafter, or by cont<strong>in</strong>uous LH-down-regulat<strong>in</strong>g<br />
GnRH-agonist treatment (Valiente et al., 2008a, b).<br />
Adm<strong>in</strong>istration <strong>of</strong> LH antiserum can cause acute<br />
transient reduction <strong>in</strong> progesterone secretion.<br />
(Concannon et al., 1993). LH but not FSH or prolact<strong>in</strong><br />
acutely stimulated progesterone synthesis by luteal cells<br />
<strong>in</strong> vitro. Prolact<strong>in</strong> is also clearly luteotrophic by day 12,<br />
at which time prolact<strong>in</strong> lower<strong>in</strong>g doses <strong>of</strong> dopam<strong>in</strong>e<br />
Anim. Reprod., v.6, n.1, p.172-193, Jan./Mar. 2009 179