Mechanosynthesis of lanthanum manganite
Mechanosynthesis of lanthanum manganite
Mechanosynthesis of lanthanum manganite
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
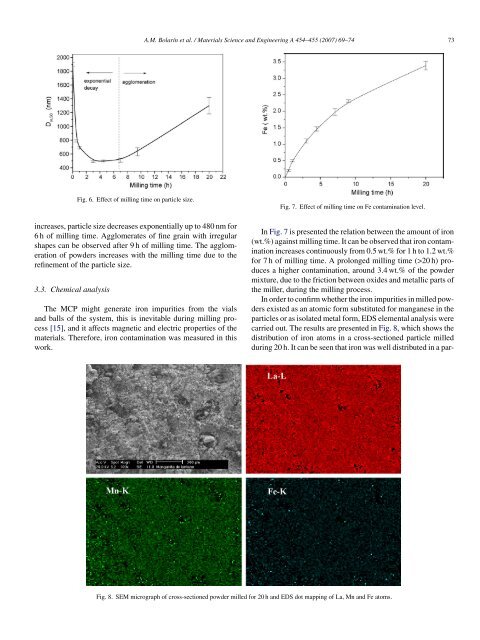
Fig. 6. Effect <strong>of</strong> milling time on particle size.<br />
increases, particle size decreases exponentially up to 480 nm for<br />
6 h <strong>of</strong> milling time. Agglomerates <strong>of</strong> fine grain with irregular<br />
shapes can be observed after 9 h <strong>of</strong> milling time. The agglomeration<br />
<strong>of</strong> powders increases with the milling time due to the<br />
refinement <strong>of</strong> the particle size.<br />
3.3. Chemical analysis<br />
The MCP might generate iron impurities from the vials<br />
and balls <strong>of</strong> the system, this is inevitable during milling process<br />
[15], and it affects magnetic and electric properties <strong>of</strong> the<br />
materials. Therefore, iron contamination was measured in this<br />
work.<br />
A.M. Bolarín et al. / Materials Science and Engineering A 454–455 (2007) 69–74 73<br />
Fig. 7. Effect <strong>of</strong> milling time on Fe contamination level.<br />
In Fig. 7 is presented the relation between the amount <strong>of</strong> iron<br />
(wt.%) against milling time. It can be observed that iron contamination<br />
increases continuously from 0.5 wt.% for 1 h to 1.2 wt.%<br />
for 7 h <strong>of</strong> milling time. A prolonged milling time (>20 h) produces<br />
a higher contamination, around 3.4 wt.% <strong>of</strong> the powder<br />
mixture, due to the friction between oxides and metallic parts <strong>of</strong><br />
the miller, during the milling process.<br />
In order to confirm whether the iron impurities in milled powders<br />
existed as an atomic form substituted for manganese in the<br />
particles or as isolated metal form, EDS elemental analysis were<br />
carried out. The results are presented in Fig. 8, which shows the<br />
distribution <strong>of</strong> iron atoms in a cross-sectioned particle milled<br />
during 20 h. It can be seen that iron was well distributed in a par-<br />
Fig. 8. SEM micrograph <strong>of</strong> cross-sectioned powder milled for 20 h and EDS dot mapping <strong>of</strong> La, Mn and Fe atoms.