TEMA 10-Disoluciones
TEMA 10-Disoluciones
TEMA 10-Disoluciones
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
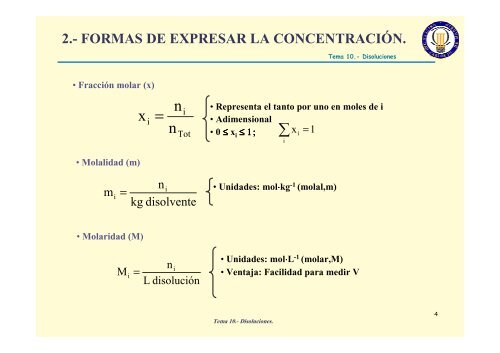
2.- FORMAS DE EXPRESAR LA CONCENTRACIÓN.<br />
• Fracción molar (x)<br />
• Molalidad (m)<br />
m<br />
i =<br />
• Molaridad (M)<br />
M<br />
x =<br />
i<br />
n<br />
n<br />
Tot<br />
ni<br />
kg disolvente<br />
i =<br />
ni<br />
L disolución<br />
i<br />
• Unidades: mol⋅kg -1 (molal,m)<br />
Tema <strong>10</strong>.- <strong>Disoluciones</strong>.<br />
Tema <strong>10</strong>.- <strong>Disoluciones</strong><br />
• Representa el tanto por uno en moles de i<br />
• Adimensional<br />
• 0 ≤ xi ≤ 1; xi<br />
= 1<br />
∑<br />
i<br />
• Unidades: mol⋅L -1 (molar,M)<br />
• Ventaja: Facilidad para medir V<br />
4