PIA Química I
PIA_BrendaNataliaFerialPerea/Grupo 108/ N.L.8
PIA_BrendaNataliaFerialPerea/Grupo 108/ N.L.8
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
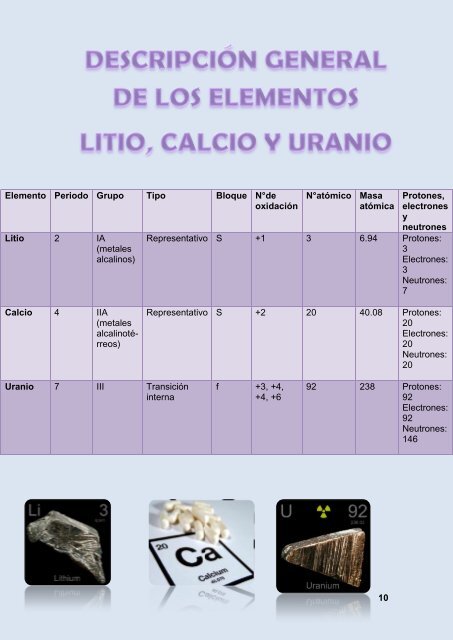
Elemento Periodo Grupo Tipo Bloque N°de<br />
oxidación<br />
Litio 2 IA<br />
(metales<br />
alcalinos)<br />
N°atómico Masa<br />
atómica<br />
Protones,<br />
electrones<br />
y<br />
neutrones<br />
Representativo S +1 3 6.94 Protones:<br />
3<br />
Electrones:<br />
3<br />
Neutrones:<br />
7<br />
Calcio 4 IIA<br />
(metales<br />
alcalinotérreos)<br />
Representativo S +2 20 40.08 Protones:<br />
20<br />
Electrones:<br />
20<br />
Neutrones:<br />
20<br />
Uranio 7 III Transición<br />
interna<br />
f +3, +4,<br />
+4, +6<br />
92 238 Protones:<br />
92<br />
Electrones:<br />
92<br />
Neutrones:<br />
146<br />
10