Nomenclature handout
Nomenclature handout
Nomenclature handout
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
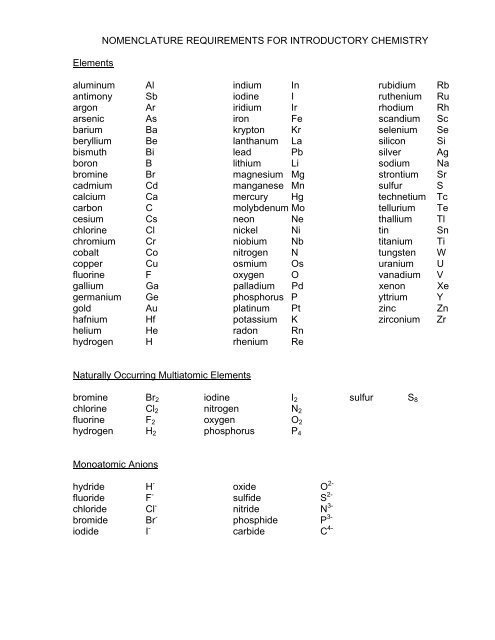
Elements<br />
NOMENCLATURE REQUIREMENTS FOR INTRODUCTORY CHEMISTRY<br />
aluminum Al indium In rubidium Rb<br />
antimony Sb iodine I ruthenium Ru<br />
argon Ar iridium Ir rhodium Rh<br />
arsenic As iron Fe scandium Sc<br />
barium Ba krypton Kr selenium Se<br />
beryllium Be lanthanum La silicon Si<br />
bismuth Bi lead Pb silver Ag<br />
boron B lithium Li sodium Na<br />
bromine Br magnesium Mg strontium Sr<br />
cadmium Cd manganese Mn sulfur S<br />
calcium Ca mercury Hg technetium Tc<br />
carbon C molybdenum Mo tellurium Te<br />
cesium Cs neon Ne thallium Tl<br />
chlorine Cl nickel Ni tin Sn<br />
chromium Cr niobium Nb titanium Ti<br />
cobalt Co nitrogen N tungsten W<br />
copper Cu osmium Os uranium U<br />
fluorine F oxygen O vanadium V<br />
gallium Ga palladium Pd xenon Xe<br />
germanium Ge phosphorus P yttrium Y<br />
gold Au platinum Pt zinc Zn<br />
hafnium Hf potassium K zirconium Zr<br />
helium He radon Rn<br />
hydrogen H rhenium Re<br />
Naturally Occurring Multiatomic Elements<br />
bromine Br2 iodine I2 sulfur S8<br />
chlorine Cl2 nitrogen N2<br />
fluorine F2 oxygen O2<br />
hydrogen H2 phosphorus P4<br />
Monoatomic Anions<br />
hydride H -<br />
fluoride F -<br />
chloride Cl -<br />
bromide Br -<br />
iodide I -<br />
oxide O 2-<br />
sulfide S 2-<br />
nitride N 3-<br />
phosphide P 3-<br />
carbide C 4-
Polyatomic Anions<br />
+<br />
ammonium NH4<br />
-<br />
acetate C2H3O2<br />
2-<br />
carbonate<br />
hydrogen carbonate<br />
perchlorate<br />
chlorate<br />
chlorite<br />
hypochlorite<br />
CO3<br />
-<br />
HCO3<br />
-<br />
ClO4<br />
-<br />
ClO3<br />
-<br />
ClO2<br />
ClO -<br />
perbromate<br />
bromate<br />
bromite<br />
hypobromite<br />
-<br />
BrO4<br />
-<br />
BrO3<br />
-<br />
BrO2<br />
BrO -<br />
periodate<br />
iodate<br />
ioditehypoiodite<br />
-<br />
IO4<br />
-<br />
IO3<br />
-<br />
IO2<br />
IO -<br />
permanganate<br />
-<br />
MnO4<br />
Binary Acids<br />
nitrate<br />
nitrite<br />
chromate<br />
dichromate<br />
cyanide<br />
-<br />
NO3<br />
-<br />
NO2<br />
2-<br />
CrO4<br />
2-<br />
Cr2O7<br />
CN -<br />
hydrogen sulfate<br />
sulfate<br />
sulfite<br />
hydrogen sulfite<br />
hydroxide<br />
-<br />
HSO4<br />
2-<br />
SO4<br />
2-<br />
SO3<br />
-<br />
HSO3<br />
OH -<br />
phosphate<br />
hydrogen phosphate<br />
dihydrogen phosphate<br />
phosphite<br />
hydrogen phosphite<br />
dihydrogen phosphite<br />
3-<br />
PO4<br />
2-<br />
HPO4<br />
-<br />
H2PO4<br />
3-<br />
PO3<br />
2-<br />
HPO3<br />
-<br />
H2PO3<br />
hydrofluoric acid HF hydroiodic acid HI<br />
hydrochloric acid HCl hydrosulfuric acid H2S<br />
hydrobromic acid HBr<br />
Oxyacids<br />
acetic acid HC2H3O2 permanganic acid HMnO4<br />
carbonic acid H2CO3 chromic acid H2CrO4<br />
nitric acid HNO3 phosphoric acid H3PO4<br />
nitrous acid HNO2 phosphorous acid H3PO3<br />
perchloric acid HClO4 sulfuric acid H2SO4<br />
chloric acid HClO3 sulfurous acid H2SO3<br />
chlorous acid HClO2 hydrocyanic acid HCN<br />
hypochlorous acid HClO<br />
Also include all other halogen acids in this list.
Greek Prefixes<br />
1 mono 4 tetra 7 hepta 10 deca<br />
2 di 5 penta 8 octa<br />
3 tri 6 hexa 9 nona<br />
Oxides of the Main Group Elements<br />
dinitrogen monoxide N2O sulfur trioxide SO3<br />
nitrogen monoxide NO diphosphorus pentoxide P2O5<br />
dinitrogen trioxide N2O3 carbon monoxide CO<br />
nitrogen dioxide NO2 carbon dioxide CO2<br />
dinitrogen tetraoxide N2O4 silicon dioxide SiO2<br />
dinitrogen pentoxide N2O5 chlorine dioxide ClO2<br />
This is only a representation of this type of nomenclature.<br />
Metals with fixed oxidation states<br />
aluminum Al 3+<br />
Al<br />
cadmium Cd 2+<br />
Cd<br />
calcium Ca 2+<br />
Ca<br />
lithium Li +<br />
Li<br />
magnesium Mg 2+ Mg<br />
potassium K +<br />
K<br />
silver Ag +<br />
Ag<br />
sodium Na +<br />
Na<br />
zinc Zn 2+<br />
Zn<br />
Metals with variable oxidation states<br />
chromium Cr 2+ Cr 3+ Cr 6+<br />
Cr(II) Cr(III) Cr(VI)<br />
cobalt Co 2+ Co 3+<br />
Co(II) Co(III)<br />
copper Cu + Cu 2+<br />
Cu(I) Cu(II)<br />
gold Au + Au 3+<br />
Au(I) Au(III)<br />
iron Fe 2+ Fe 3+<br />
Fe(II) Fe(III)<br />
lead Pb 2+ Pb 4+<br />
Pb(II) Pb(IV)<br />
manganese Mn 2+ Mn 3+ Mn 4+ Mn 7+ Mn(II) Mn(III) Mn(IV) Mn(VII)<br />
mercury<br />
nickel<br />
2+ 2+<br />
Hg2 Hg<br />
Ni<br />
Hg(I) Hg(II)<br />
2+ Ni 3+<br />
Ni(II) Ni(III)<br />
tin Sn 2+ Sn 4+<br />
Sn(II) Sn(IV)<br />
uranium U 3+ U 4+ U 5+ U 6+ U(III) U(IV) U(V) U(VI)
Hydrates<br />
Use the Greek prefixes when naming hydrates to indicate the number of water molecules<br />
associated with each compound.<br />
CoSO4 • H2O cobalt(II) sulfate monohydrate<br />
BaI2 • 2 H2O barium iodide dihydrate<br />
Au(CN)3 • 3 H2O gold(III) cyanide trihydrate<br />
FeI2 • 4 H2O iron(II) iodide tetrahydrate<br />
MnSO4 • 5 H2O manganese(II) sulfate pentahydrate<br />
Cd(MnO4)2 • 6 H2O cadmium permanganate hexahydrate<br />
ZnSO4 • 7 H2O zinc sulfate heptahydrate<br />
Mg3(PO4)2 • 8 H2O magnesium phosphate octahydrate<br />
Al(BrO3)3 • 9 H2O aluminum bromate nonahydrate<br />
Pb(C2H3O2)2 • 10 H2O lead(II) acetate decahydrate




![Graduate Bulletin [PDF] - MFC home page - Appalachian State ...](https://img.yumpu.com/50706615/1/190x245/graduate-bulletin-pdf-mfc-home-page-appalachian-state-.jpg?quality=85)











