I0N34a THERMODYNAMICA Oefenzitting 4 - bio-ingenieur - home
I0N34a THERMODYNAMICA Oefenzitting 4 - bio-ingenieur - home
I0N34a THERMODYNAMICA Oefenzitting 4 - bio-ingenieur - home
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>I0N34a</strong> Thermodynamica 2 de studiefase <strong>bio</strong>-<strong>ingenieur</strong>swetenschappen<br />
P4 = P3V3 γ V4 -γ = 2,64 10 5 Pa<br />
W = -1781 J<br />
T4 = 792 K<br />
4→1: geen arbeid<br />
Netto arbeid: + 1044 – 609,2 – 1781 = -1346 J in één cyclus.<br />
Warmte opname in stap 2→3:<br />
Q nC T =2123 J (T stijgt van 953 naar 1906 K)<br />
p<br />
Warmte-afgifte in stap 4→1:<br />
Q nC T = - 782 J<br />
v<br />
b)<br />
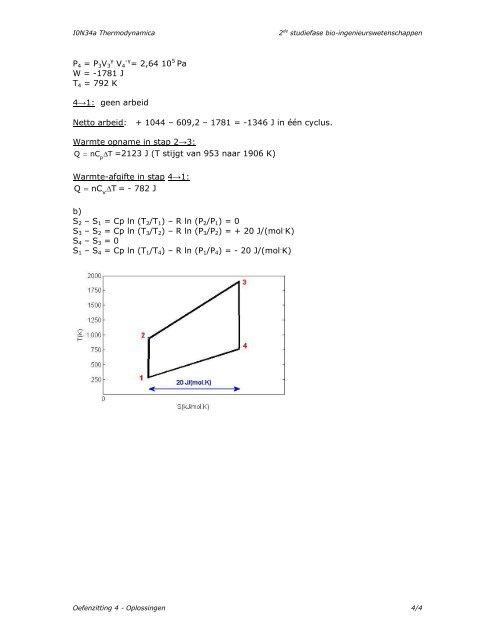
S2 – S1 = Cp ln (T2/T1) – R ln (P2/P1) = 0<br />
S3 – S2 = Cp ln (T3/T2) – R ln (P3/P2) = + 20 J/(mol . K)<br />
S4 – S3 = 0<br />
S1 – S4 = Cp ln (T1/T4) – R ln (P1/P4) = - 20 J/(mol . K)<br />
<strong>Oefenzitting</strong> 4 - Oplossingen 4/4