Clique aqui para download - senai
Clique aqui para download - senai
Clique aqui para download - senai
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
generally delocalized because of mode-mode coupling<br />
effects or Fermi resonances between vibrational modes<br />
and overtones. Therefore, a vibrational normal mode<br />
cannot be exclusively assigned to an individual bond or a<br />
particular structural unit. A solution to this problem will<br />
be presented in the next section.<br />
Results and Discussions<br />
Cremer and co-workers have recently demonstrated<br />
that for each molecule both a set of normal vibrational<br />
modes and a unique set of local vibrational modes<br />
exists. 4 The force constants of the local bond stretching<br />
modes reliably describe the strength of the bonds of a<br />
molecule. 5 Hence they can be used to investigate one of<br />
the <strong>para</strong>digm of chemistry which predicts that the shorter<br />
bond of a given type is always the stronger bond. This has<br />
been found to be fulfi lled in many cases on qualitative<br />
grounds, 6 however there exists no theoretical proof of the<br />
<strong>para</strong>digm, which has to do with the elusiveness of the<br />
chemical bond and the resulting diffi culty of defi ning the<br />
bond strength.<br />
In the following, we will demonstrate that it is<br />
not generally true that the shorter bond is the stronger<br />
bond. For this purpose, we consider the strength of the<br />
NF bond in the series NH 2 F (1), NHF 2 (2), and NF 3<br />
(3). Since these molecules are closely related, one can<br />
assume that, according to the above <strong>para</strong>digm, the NF<br />
bond becomes stronger with decreasing NF bond length.<br />
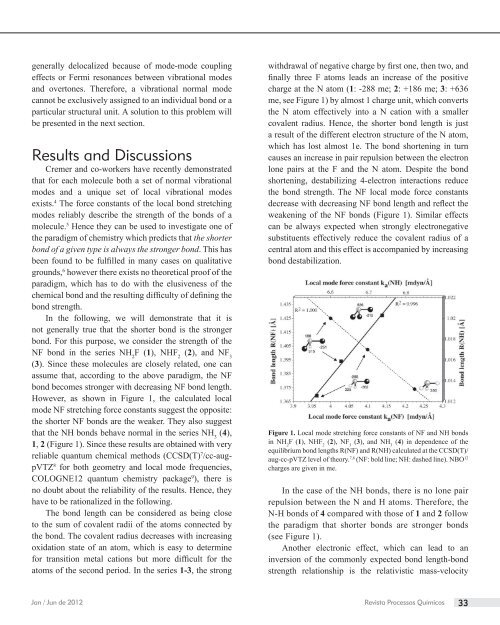
However, as shown in Figure 1, the calculated local<br />
mode NF stretching force constants suggest the opposite:<br />
the shorter NF bonds are the weaker. They also suggest<br />
that the NH bonds behave normal in the series NH 3 (4),<br />
1, 2 (Figure 1). Since these results are obtained with very<br />
reliable quantum chemical methods (CCSD(T) 7 /cc-augpVTZ<br />
8 for both geometry and local mode frequencies,<br />
COLOGNE12 quantum chemistry package 9 ), there is<br />
no doubt about the reliability of the results. Hence, they<br />
have to be rationalized in the following.<br />
The bond length can be considered as being close<br />
to the sum of covalent radii of the atoms connected by<br />
the bond. The covalent radius decreases with increasing<br />
oxidation state of an atom, which is easy to determine<br />
for transition metal cations but more diffi cult for the<br />
atoms of the second period. In the series 1-3, the strong<br />
withdrawal of negative charge by fi rst one, then two, and<br />
fi nally three F atoms leads an increase of the positive<br />
charge at the N atom (1: -288 me; 2: +186 me; 3: +636<br />
me, see Figure 1) by almost 1 charge unit, which converts<br />
the N atom effectively into a N cation with a smaller<br />
covalent radius. Hence, the shorter bond length is just<br />
a result of the different electron structure of the N atom,<br />
which has lost almost 1e. The bond shortening in turn<br />
causes an increase in pair repulsion between the electron<br />
lone pairs at the F and the N atom. Despite the bond<br />
shortening, destabilizing 4-electron interactions reduce<br />
the bond strength. The NF local mode force constants<br />
decrease with decreasing NF bond length and refl ect the<br />
weakening of the NF bonds (Figure 1). Similar effects<br />
can be always expected when strongly electronegative<br />
substituents effectively reduce the covalent radius of a<br />
central atom and this effect is accompanied by increasing<br />
bond destabilization.<br />
Figure 1. Local mode stretching force constants of NF and NH bonds<br />
in NH 2 F (1), NHF 2 (2), NF 3 (3), and NH 3 (4) in dependence of the<br />
equilibrium bond lengths R(NF) and R(NH) calculated at the CCSD(T)/<br />
aug-cc-pVTZ level of theory. 7,8 (NF: bold line; NH: dashed line). NBO 12<br />
charges are given in me.<br />
In the case of the NH bonds, there is no lone pair<br />
repulsion between the N and H atoms. Therefore, the<br />
N-H bonds of 4 compared with those of 1 and 2 follow<br />
the <strong>para</strong>digm that shorter bonds are stronger bonds<br />
(see Figure 1).<br />
Another electronic effect, which can lead to an<br />
inversion of the commonly expected bond length-bond<br />
strength relationship is the relativistic mass-velocity<br />
Jan / Jun de 2012 Revista Processos Químicos 33