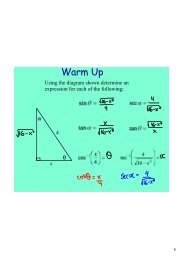
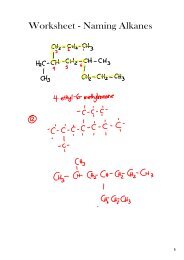Answers Physical Science 10 Chemistry Exam Review #2
Answers Physical Science 10 Chemistry Exam Review #2
Answers Physical Science 10 Chemistry Exam Review #2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1. Write the names of the following compounds:a) CO 2 carbon dioxideb) N 2 O 5 dinitrogen pentaoxidec) PF 5 phosphorous pentafluoride<strong>Answers</strong><strong>Physical</strong> <strong>Science</strong> <strong>10</strong><strong>Chemistry</strong> <strong>Exam</strong> <strong>Review</strong> <strong>#2</strong>d) NH 3 ammonia or nitrogen trihydridee) CCl 4 carbon tetrachloride2. Write the formulas for the following compounds:a) dinitrogen monoxide N 2 Ob) diphosphorous hexabromide P 2 Br 6c) sulfur dioxide SO 2d) silicon tetrahydride SiH 4e) nitrogen diselenide NSe 23. Balance the following equationsa) 8 Br 2 + 8 K 2 S → 16 KBr + S 8b) 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 Oc) 2 NaCl → 2 Na + Cl 2d) Na 2 CO 3 + CaCl 2 → CaCO 3 + 2 NaCle) 2 Fe 2 O 3 → 4 Fe + 3 O 2f) 2 Al + 6 HCl → 3 H 2 + 2 AlCl 3g) Au + HNO 3 + 3 HCl → AuCl 3 + NO + 2 H 2 O
4. Write the names of the following compounds:a) LiCl lithium chlorideb) Mg 3 P 2 magnesium phosphidec) Ba(NO 3 ) barium nitrated) K 3 PO 4 potassium phosphatee) CsOH cesium hydroxidef) Ca 3 (PO 4 ) 2 calcium phosphateg) FeO iron (II) oxideh) PbO 2 lead (IV) oxide5. Write the formulas for the following compounds:a) barium hydroxide Ba(OH) 2b) iron (II) fluoride FeF 2c) calcium sulfide Ca 2 Sd) copper (I) sulfate Cu 2 SO 4e) cobalt (III) iodide CoI 3f) aluminum oxide Al 2 O 3g) sodium carbonate Na 2 CO 3h) iron (III) nitrate Fe(NO 3)3