formula brută, formula moleculară, formula structurală
formula brută, formula moleculară, formula structurală
formula brută, formula moleculară, formula structurală
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
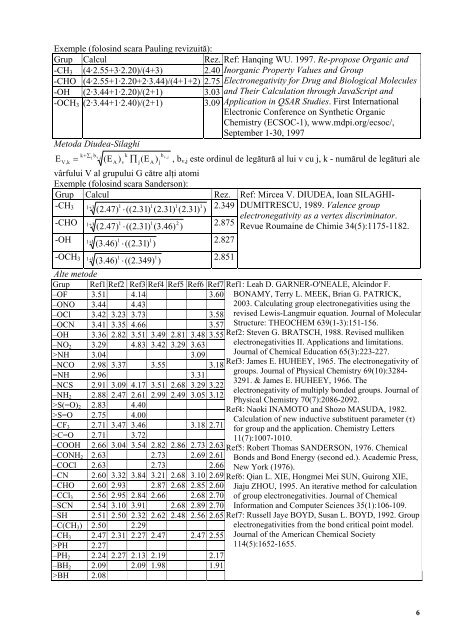
Exemple (folosind scara Pauling revizuită):<br />
Grup Calcul Rez. Ref: Hanqing WU. 1997. Re-propose Organic and<br />
-CH3 (4·2.55+3·2.20)/(4+3) 2.40 Inorganic Property Values and Group<br />
-CHO (4·2.55+1·2.20+2·3.44)/(4+1+2) 2.75 Electronegativity for Drug and Biological Molecules<br />
-OH (2·3.44+1·2.20)/(2+1) 3.03 and Their Calculation through JavaScript and<br />
-OCH3 (2·3.44+1·2.40)/(2+1)<br />
Metoda Diudea-Silaghi<br />
3.09 Application in QSAR Studies. First International<br />
Electronic Conference on Synthetic Organic<br />
Chemistry (ECSOC-1), www.mdpi.org/ecsoc/,<br />
September 1-30, 1997<br />
E<br />
V , k<br />
k+ ∑ j bv<br />
, j k<br />
bv<br />
, j<br />
( EA<br />
) v ∏ j(<br />
EA<br />
) j<br />
= , bv,j este ordinul de legătură al lui v cu j, k - numărul de legături ale<br />
vârfului V al grupului G către alţi atomi<br />
Exemple (folosind scara Sanderson):<br />
Grup Calcul Rez.<br />
-CH3 1+<br />
3 1<br />
1 1 1<br />
( 2.<br />
47)<br />
⋅((<br />
2.<br />
31)<br />
( 2.<br />
31)<br />
( 2.<br />
31)<br />
)<br />
2.349<br />
Ref: Mircea V. DIUDEA, Ioan SILAGHI-<br />
DUMITRESCU, 1989. Valence group<br />
electronegativity as a vertex discriminator.<br />
Revue Roumaine de Chimie 34(5):1175-1182.<br />
-CHO 1+<br />
3 1<br />
1 2<br />
( 2.<br />
47)<br />
⋅ (( 2.<br />
31)<br />
( 3.<br />
46)<br />
)<br />
2.875<br />
-OH 1+<br />
1 1<br />
1<br />
( 3.<br />
46)<br />
⋅ (( 2.<br />
31)<br />
)<br />
2.827<br />
-OCH3 1+<br />
1 1<br />
1<br />
( 3.<br />
46)<br />
⋅((<br />
2.<br />
349)<br />
)<br />
2.851<br />
Alte metode<br />
Grup Ref1 Ref2 Ref3 Ref4 Ref5 Ref6 Ref7 Ref1: Leah D. GARNER-O'NEALE, Alcindor F.<br />
–OF 3.51 4.14 3.60 BONAMY, Terry L. MEEK, Brian G. PATRICK,<br />
–ONO 3.44 4.43<br />
2003. Calculating group electronegativities using the<br />
–OCl 3.42 3.23 3.73 3.58 revised Lewis-Langmuir equation. Journal of Molecular<br />
–OCN 3.41 3.35 4.66 3.57 Structure: THEOCHEM 639(1-3):151-156.<br />
–OH 3.36 2.82 3.51 3.49 2.81 3.48 3.55 Ref2: Steven G. BRATSCH, 1988. Revised mulliken<br />
–NO2 3.29 4.83 3.42 3.29 3.63 electronegativities II. Applications and limitations.<br />
>NH 3.04 3.09 Journal of Chemical Education 65(3):223-227.<br />
–NCO 2.98 3.37 3.55 3.18<br />
Ref3: James E. HUHEEY, 1965. The electronegativity of<br />
=NH 2.96 3.31<br />
groups. Journal of Physical Chemistry 69(10):3284-<br />
3291. & James E. HUHEEY, 1966. The<br />
–NCS 2.91 3.09 4.17 3.51 2.68 3.29 3.22<br />
electronegativity of multiply bonded groups. Journal of<br />
–NH2 2.88 2.47 2.61 2.99 2.49 3.05 3.12<br />
Physical Chemistry 70(7):2086-2092.<br />
>S(=O)2 2.83 4.40<br />
Ref4: Naoki INAMOTO and Shozo MASUDA, 1982.<br />
>S=O 2.75 4.00<br />
Calculation of new inductive substituent parameter (τ)<br />
–CF3 2.71 3.47 3.46 3.18 2.71<br />
for group and the application. Chemistry Letters<br />
>C=O 2.71 3.72<br />
11(7):1007-1010.<br />
–COOH 2.66 3.04 3.54 2.82 2.86 2.73 2.63 Ref5: Robert Thomas SANDERSON, 1976. Chemical<br />
–CONH2 2.63 2.73 2.69 2.61 Bonds and Bond Energy (second ed.). Academic Press,<br />
–COCl 2.63 2.73 2.66 New York (1976).<br />
–CN 2.60 3.32 3.84 3.21 2.68 3.10 2.69 Ref6: Qian L. XIE, Hongmei Mei SUN, Guirong XIE,<br />
–CHO 2.60 2.93 2.87 2.68 2.85 2.60 Jiaju ZHOU, 1995. An iterative method for calculation<br />
–CCl3 2.56 2.95 2.84 2.66 2.68 2.70 of group electronegativities. Journal of Chemical<br />
–SCN 2.54 3.10 3.91 2.68 2.89 2.70 Information and Computer Sciences 35(1):106-109.<br />
–SH 2.51 2.50 2.32 2.62 2.48 2.56 2.65 Ref7: Russell Jaye BOYD, Susan L. BOYD, 1992. Group<br />
–C(CH3) 2.50 2.29<br />
electronegativities from the bond critical point model.<br />
–CH3<br />
>PH<br />
2.47 2.31 2.27 2.47<br />
2.27<br />
2.47 2.55 Journal of the American Chemical Society<br />
114(5):1652-1655.<br />
–PH2 2.24 2.27 2.13 2.19 2.17<br />
–BH2 2.09 2.09 1.98 1.91<br />
>BH 2.08<br />
6