"Sonochemistry" in Comprehensive Coordination Chemistry 2
"Sonochemistry" in Comprehensive Coordination Chemistry 2
"Sonochemistry" in Comprehensive Coordination Chemistry 2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1.41<br />
CCC.0005 Sonochemistry<br />
K. S. SUSLICK<br />
University of Ill<strong>in</strong>ois at Urbana-Champaign, Urbana, IL, USA<br />
1.41.1 INTRODUCTION 1<br />
1.41.1.1 Acoustic Cavitation 2<br />
1.41.1.2 Microjet Formation Dur<strong>in</strong>g Cavitation at Liquid–Solid Interfaces 3<br />
1.41.2 SONOLUMINESCENCE 4<br />
1.41.2.1 Types of Sonolum<strong>in</strong>escence 4<br />
1.41.2.2 Spectroscopic Probes of Cavitation Conditions 5<br />
1.41.3 SONOCHEMISTRY 5<br />
1.41.3.1 Homogeneous Sonochemistry: BondBreak<strong>in</strong>g andRadical Formation 6<br />
1.41.3.2 Applications of Sonochemistry to Materials Synthesis 6<br />
1.41.3.3 Heterogeneous Sonochemistry: Reactions of Solids with Liquids 7<br />
1.41.4 CONCLUSIONS 9<br />
1.41.5 REFERENCES 9<br />
SECCCC01046.1.41.1 1.41.1 INTRODUCTION<br />
01046<br />
CCC01046.0010 Ultrasonic irradiation of liquids causes high-energy chemical reactions to occur, often with the<br />
emission of light.[bib1 bib2 bib3] 1–3 The orig<strong>in</strong> of sonochemistry andsonolum<strong>in</strong>escence is acoustic<br />
cavitation: the formation, growth, andimplosive collapse of bubbles <strong>in</strong> liquids irradiatedwith<br />
high-<strong>in</strong>tensity sound. The collapse of bubbles caused by cavitation produces <strong>in</strong>tense local heat<strong>in</strong>g<br />
andhigh pressures, with very short lifetimes. In clouds of cavitat<strong>in</strong>g bubbles, these hot-spots[bib4<br />
bib5 bib6] 4–6 have equivalent temperatures of roughly 5,000 K, pressures of about 1,000 atm, and<br />
heat<strong>in</strong>g andcool<strong>in</strong>g rates above 10 10 Ks 1 . In s<strong>in</strong>gle bubble cavitation, conditions may be even<br />
more extreme. Thus, cavitation can create extraord<strong>in</strong>ary physical and chemical conditions <strong>in</strong><br />
otherwise coldliquids.<br />
CCC01046.0015 When liquids that conta<strong>in</strong> solids are irradiated with ultrasound, related phenomena can occur.<br />
When cavitation occurs near an extended solid surface, cavity collapse is non-spherical and drives<br />
7<br />
high-speedjets of liquid<strong>in</strong>to the surface. [bib7] These jets andassociatedshock waves can cause<br />
substantial surface damage and expose fresh, highly heated surfaces. Ultrasonic irradiation of<br />
liquid–powder suspensions produces another effect: high-velocity <strong>in</strong>ter-particle collisions. Cavitation<br />
andthe shockwaves it creates <strong>in</strong> a slurry can accelerate solidparticles to high velocities.[bib8] 8<br />
The resultant collisions are capable of <strong>in</strong>duc<strong>in</strong>g dramatic changes <strong>in</strong> surface morphology, composition,<br />
andreactivity.[bib9] 9<br />
CCC01046.0020 Sonochemistry can be roughly divided <strong>in</strong>to categories based on the nature of the cavitation<br />
event: homogeneous sonochemistry of liquids, heterogeneous sonochemistry of liquid–liquid or<br />
liquid–solid systems, and sonocatalysis (which overlaps the first two). In some cases, ultrasonic<br />
irradiation can <strong>in</strong>crease reactivity by nearly a million-fold.[bib10] 10 Because cavitation can only<br />
occur <strong>in</strong> liquids, chemical reactions are not generally seen <strong>in</strong> the ultrasonic irradiation of solids or<br />
solid–gas systems.<br />
CCC01046.0025 Sonolum<strong>in</strong>escence <strong>in</strong> general may be considered a special case of homogeneous sonochemistry;<br />
however, recent discoveries <strong>in</strong> this field have heightened <strong>in</strong>terest <strong>in</strong> the phenomenon <strong>in</strong> and by<br />
1
2 Sonochemistry<br />
itself.[bib11 bib12] 11,12 Under conditions where an isolated, s<strong>in</strong>gle bubble undergoes cavitation,<br />
recent studies on the duration of the sonolum<strong>in</strong>escence flash suggest that a shock wave may be<br />
createdwith<strong>in</strong> the collaps<strong>in</strong>g bubble, with the capacity to generate truly enormous temperatures<br />
andpressures with<strong>in</strong> the gas.<br />
secCCC01046.1.41.1.1 1.41.1.1 Acoustic Cavitation<br />
CCC01046.0030 The chemical effects of ultrasound do not arise from a direct <strong>in</strong>teraction with molecular species.<br />
Ultrasoundspans the frequencies of roughly 15 kHz to 1 GHz. With soundvelocities <strong>in</strong> liquids<br />
typically about 1,500 m s 1 , acoustic wavelengths range from roughly 10 to 10 4 cm. These are not<br />
molecular dimensions. Consequently, no direct coupl<strong>in</strong>g of the acoustic field with chemical species<br />
on a molecular level can account for sonochemistry or sonolum<strong>in</strong>escence.<br />
CCC01046.0035 Instead, sonochemistry and sonolum<strong>in</strong>escence derive pr<strong>in</strong>cipally from acoustic cavitation,<br />
which serves as an effective means of concentrat<strong>in</strong>g the diffuse energy of sound. Compression<br />
of a gas generates heat. When the compression of bubbles occurs dur<strong>in</strong>g cavitation, it is more<br />
rapidthan thermal transport, which generates a short-lived, localizedhot-spot. Rayleigh’s early<br />
descriptions of a mathematical model for the collapse of cavities <strong>in</strong> <strong>in</strong>compressible liquids<br />
predicted enormous local temperatures and pressures.[bib13] 13 Ten years later, Richards and<br />
Loomis reportedthe first chemical andbiological effects of ultrasound.[bib14] 14<br />
CCC01046.0040 If the acoustic pressure amplitude of a propagat<strong>in</strong>g acoustic wave is relatively large (greater<br />
than 0.5 MPa), local <strong>in</strong>homogeneities <strong>in</strong> the liquidcan give rise to the rapidgrowth of a<br />
nucleation site <strong>in</strong>to a cavity of macroscopic dimensions, primarily filled with vapor. Such a<br />
bubble is <strong>in</strong>herently unstable, andits subsequent collapse can result <strong>in</strong> an enormous concentration<br />
of energy ( [figCCC01046.1] Figure 1). This violent cavitation event is generally termed‘‘transient<br />
cavitation.’’ A normal consequence of this unstable growth andsubsequent collapse is that the<br />
cavitation bubble itself is destroyed. Gas-filled remnants from the collapse, however, may give rise<br />
to re<strong>in</strong>itiation of the process.<br />
CCC01046.0045 A variety of devices have been used for ultrasonic irradiation of solutions. There are three<br />
general designs <strong>in</strong> use presently: the ultrasonic clean<strong>in</strong>g bath, the direct immersion ultrasonic<br />
horn, andflow reactors. The orig<strong>in</strong>at<strong>in</strong>g source of the ultrasoundis generally a piezoceramic<br />
material that is subjectedto a high AC voltage with an ultrasonic frequency (typically 15 to<br />
50 kHz). The vibrat<strong>in</strong>g source is attachedto the wall of a clean<strong>in</strong>g bath, to an amplify<strong>in</strong>g horn, or<br />
to the outer surfaces of a flow-through tube or diaphragm. The ultrasonic clean<strong>in</strong>g bath is clearly<br />
the most accessible source of laboratory ultrasoundandhas been usedsuccessfully for a variety of<br />
liquid–solid heterogeneous sonochemical studies. The low <strong>in</strong>tensity available <strong>in</strong> these devices<br />
( 1Wcm 2 ), however, means that even <strong>in</strong> the case of heterogeneous sonochemistry, an ultrasonic<br />
clean<strong>in</strong>g bath must be viewedas an apparatus of limitedcapability. The most <strong>in</strong>tense andreliable<br />
source of ultrasoundgenerally used<strong>in</strong> the chemical laboratory is the direct immersion ultrasonic<br />
Acoustic<br />
pressure<br />
Liquid<br />
density<br />
Bubble radius (mm)<br />
150<br />
100<br />
50<br />
+<br />
–<br />
Growth<br />
Implosion<br />
Shockwave (?)<br />
Rapid<br />
0<br />
Formation<br />
Hot spot quench<strong>in</strong>g<br />
0 100 200 300 400 500<br />
Time (µs)<br />
figCCC01046.1 Figure 1 Transient acoustic cavitation: the orig<strong>in</strong> of sonochemistry andsonolum<strong>in</strong>escence.
Cool<strong>in</strong>g<br />
bath<br />
Sonochemistry 3<br />
Power supply<br />
Piezoelectric<br />
transducer<br />
Titanium horn<br />
Collar & O-r<strong>in</strong>gs<br />
Gas <strong>in</strong>let / outlet<br />
Glass cell<br />
Reaction<br />
solution<br />
figCCC01046.2 Figure 2 A typical sonochemical apparatus with direct immersion ultrasonic horn. Ultrasound can be easily<br />
<strong>in</strong>troduced <strong>in</strong>to a chemical reaction with good control of temperature and ambient atmosphere.<br />
horn (50 to 500 W cm 2 ), as shown <strong>in</strong> [figCCC01046.2] Figure 2, which can be usedfor work under<br />
either <strong>in</strong>ert or reactive atmospheres or at moderate pressures (
4 Sonochemistry<br />
before ultrasound<br />
100µm<br />
heterogeneous reactions. The erosion of metals by cavitation generates newly exposed, highly<br />
heatedsurfaces.<br />
CCC01046.0065 A solidsurface several times larger than the resonance bubble size is necessary to <strong>in</strong>duce<br />
distortions dur<strong>in</strong>g bubble collapse. For ultrasound of 20 kHz, damage associated with jet<br />
formation cannot occur if the solidparticles are smaller than 200 m. In these cases, however,<br />
the shockwaves createdby homogeneous cavitation can create high-velocity <strong>in</strong>terparticle collisions.[bib8<br />
bib9] 8,9 Suslick andco-workers have foundthat the turbulent flow andshockwaves<br />
produced by <strong>in</strong>tense ultrasound can drive metal particles together at sufficiently high speeds to<br />
<strong>in</strong>duce effective melt<strong>in</strong>g <strong>in</strong> direct collisions and the abrasion of surface crystallites <strong>in</strong> glanc<strong>in</strong>g<br />
impacts ([figCCC01046.3] Figure 3). A series of transition metal powders were used to probe the<br />
maximum temperatures and speeds reached dur<strong>in</strong>g <strong>in</strong>terparticle collisions. Us<strong>in</strong>g the irradiation<br />
of Cr, Mo, and W powders <strong>in</strong> decane at 20 kHz and 50 W cm 2 , agglomeration andessentially a<br />
localizedmelt<strong>in</strong>g occurs for the first two metals, but not the third. On the basis of the melt<strong>in</strong>g<br />
po<strong>in</strong>ts of these metals, the effective transient temperature reachedat the po<strong>in</strong>t of impact dur<strong>in</strong>g<br />
<strong>in</strong>terparticle collisions is roughly 3,000 C (which is unrelatedto the temperature <strong>in</strong>side the hotspot<br />
of a collaps<strong>in</strong>g bubble). From the volume of the meltedregion of impact, the amount of<br />
energy generated dur<strong>in</strong>g collision was determ<strong>in</strong>ed. From this, a lower estimate of the velocity of<br />
impact is roughly one half the speedof sound. [bib8] 8 These are precisely the effects expectedon<br />
suspended particulates from cavitation-<strong>in</strong>duced shockwaves <strong>in</strong> the liquid.<br />
SECCCC01046.1.41.2 1.41.2 SONOLUMINESCENCE<br />
secCCC01046.1.41.2.1 1.41.2.1 Types of Sonolum<strong>in</strong>escence<br />
60 m<strong>in</strong>. ultrasound<br />
figCCC01046.3 Figure 3 The effect of ultrasonic irradiation on the surface morphology and particle size of Ni powder.<br />
High-velocity <strong>in</strong>terparticle collisions causedby ultrasonic irradiation of slurries are responsible for the<br />
smooth<strong>in</strong>g and removal of passivat<strong>in</strong>g oxide coat<strong>in</strong>g. Reproduced with permission.[bib9] 9<br />
CCC01046.0070 In addition to driv<strong>in</strong>g chemical reactions, ultrasonic irradiation of liquids can also produce light.<br />
Sonolum<strong>in</strong>escence was first observedfrom water <strong>in</strong> 1934 by Frenzel andSchultes. [bib15] 15 As with<br />
sonochemistry, sonolum<strong>in</strong>escence derives from acoustic cavitation. It is now generally thought<br />
that there are two separate forms of sonolum<strong>in</strong>escence: multiple-bubble sonolum<strong>in</strong>escence<br />
(MBSL) ands<strong>in</strong>gle-bubble sonolum<strong>in</strong>escence (SBSL). [bib11 bib12] 11,12 S<strong>in</strong>ce cavitation is a<br />
nucleatedprocess andliquids generally conta<strong>in</strong> large numbers of particulates that serve as nuclei,<br />
the ‘‘cavitation field’’ generated by a propagat<strong>in</strong>g or stand<strong>in</strong>g acoustic wave typically consists of<br />
very large numbers of <strong>in</strong>teract<strong>in</strong>g bubbles, distributed over an extended region of the liquid. If<br />
this cavitation is sufficiently <strong>in</strong>tense to produce sonolum<strong>in</strong>escence, then the phenomenon is<br />
MBSL.[bib1 bib16] 1,16<br />
CCC01046.0075 Under the appropriate conditions, the acoustic force on a bubble can be used to balance aga<strong>in</strong>st<br />
its buoyancy, hold<strong>in</strong>g the bubble stable <strong>in</strong> the liquid by acoustic levitation. This permits exam<strong>in</strong>ation<br />
of the dynamic characteristics of the bubble <strong>in</strong> considerable detail, from both a theoretical<br />
andan experimental perspective. Such a bubble is typically quite small comparedto an acoustic<br />
wavelength (e.g., at 20 kHz, the resonance size is approximately 150 mm). For rather specialized<br />
but easily obta<strong>in</strong>able conditions, a s<strong>in</strong>gle, stable, oscillat<strong>in</strong>g gas bubble can be forced <strong>in</strong>to such<br />
large-amplitude pulsations that it produces sonolum<strong>in</strong>escence emissions on each (and every)<br />
acoustic cycle. Such SBSL is outside the scope of this chapter.
secCCC01046.1.41.2.2 1.41.2.2 Spectroscopic Probes of Cavitation Conditions<br />
CCC01046.0080 The MBSL of both aqueous andnon-aqueous solutions is similar to the emission expectedfrom<br />
high-temperature flames; e.g. excited-state OH from water, excitedstates of C2 (d 3<br />
g -a 3 u,)<br />
from hydrocarbons (these l<strong>in</strong>es also give hydrocarbon flames their blue color), and CN excited<br />
states <strong>in</strong> the presence of a nitrogen source. For both aqueous andnon-aqueous liquids, MBSL is<br />
causedby chemical reactions of high-energy species formeddur<strong>in</strong>g cavitation by bubble collapse.<br />
Its pr<strong>in</strong>cipal source is most probably not blackbody radiation or electrical discharge. MBSL is a<br />
form of chemilum<strong>in</strong>escence.[bib16] 16<br />
CCC01046.0085 Determ<strong>in</strong>ation of the temperatures reached<strong>in</strong> a cavitat<strong>in</strong>g bubble has rema<strong>in</strong>eda difficult<br />
experimental problem. As a spectroscopic probe of the cavitation event, MBSL provides a<br />
solution. High-resolution MBSL spectra from silicone oil under Ar have been reported and<br />
analyzed.[bib5] 5 The observedemission comes from excited-state C2 andhas been modeledwith<br />
synthetic spectra as a function of rotational andvibrational temperatures. From comparison of<br />
synthetic to observedspectra, the effective cavitation temperature is 5,050 150 K.<br />
CCC01046.0090 A secondspectroscopic thermometer comes from the relative <strong>in</strong>tensities of atomic emission<br />
l<strong>in</strong>es <strong>in</strong> the sonolum<strong>in</strong>escence spectra of excited-state metal atoms produced by sonolysis of<br />
volatile Fe, Cr, andMo carbonyls. Sufficient spectral <strong>in</strong>formation about emissivities of many<br />
metal atom excitedstates are available to readily calculate emission spectra as a function of<br />
temperature. Because of this, the emission spectra of metal atoms are extensively usedby<br />
astronomers to monitor the surface temperature of stars. From comparison of calculatedspectra<br />
andthe observedMBSL spectra from metal carbonyls, another measurement of the cavitation<br />
temperature was obta<strong>in</strong>ed.[bib6] 6 The effective emission temperature from metal atom emission<br />
dur<strong>in</strong>g cavitation under argon at 20 kHz is 4,900 250 K.<br />
CCC01046.0095 The excellence of the match between the observedMBSL andthe synthetic spectra provides<br />
def<strong>in</strong>itive proof that the sonolum<strong>in</strong>escence event is a thermal, chemilum<strong>in</strong>escence process. The<br />
agreement among these spectroscopic determ<strong>in</strong>ations [bib5 bib6] 5,6 of the cavitation temperature<br />
andto that made by comparative rate thermometry of sonochemical reactions[bib4] 4 is extremely<br />
good.<br />
SECCCC01046.1.41.3 1.41.3 SONOCHEMISTRY<br />
Sonochemistry 5<br />
CCC01046.0100 In a fundamental sense, chemistry is the <strong>in</strong>teraction of energy and matter. Chemical reactions<br />
require energy <strong>in</strong> one form or another to proceed: chemistry stops as the temperature approaches<br />
absolute zero. One has only limitedcontrol, however, over the nature of this <strong>in</strong>teraction. In large<br />
part, the properties of a specific energy source determ<strong>in</strong>e the course of a chemical reaction.<br />
Ultrasonic irradiation differs from traditional energy sources (such as heat, light, or ioniz<strong>in</strong>g<br />
radiation) <strong>in</strong> duration, pressure, and energy per molecule. The immense local temperatures and<br />
pressures andthe extraord<strong>in</strong>ary heat<strong>in</strong>g andcool<strong>in</strong>g rates generatedby cavitation bubble collapse<br />
mean that ultrasoundprovides an unusual mechanism for generat<strong>in</strong>g high-energy chemistry. Like<br />
photochemistry, very large amounts of energy are <strong>in</strong>troduced <strong>in</strong> a short period of time, but it is<br />
thermal, not electronic, excitation. As <strong>in</strong> flash pyrolysis, high thermal temperatures are reached,<br />
but the duration is very much shorter (by >10 4 ) andthe temperatures are even higher (by five- to<br />
ten-fold). Similar to shock-tube chemistry or multiphoton <strong>in</strong>frared laser photolysis, cavitation<br />
heat<strong>in</strong>g is very short lived, but occurs with<strong>in</strong> condensed phases. Furthermore, sonochemistry has<br />
a high-pressure component, which suggests that one might be able to produce on a microscopic<br />
scale the same macroscopic conditions of high-temperature pressure ‘‘bomb’’ reactions or explosive<br />
shockwave synthesis <strong>in</strong> solids. Control of sonochemical reactions is subject to the same<br />
limitation that any thermal process has: the Boltzmann energy distribution means that the energy<br />
per <strong>in</strong>dividual molecule will vary widely. One does have easy control, however, over the <strong>in</strong>tensity<br />
of heat<strong>in</strong>g generatedby acoustic cavitation us<strong>in</strong>g various physical parameters (<strong>in</strong>clud<strong>in</strong>g thermal<br />
conductivity of dissolved gases, solvent vapor pressure <strong>in</strong>side the bubble, and ambient pressure).<br />
In contrast, frequency appears to be less important, at least with<strong>in</strong> the range where cavitation can<br />
occur (a few hertz to a few megahertz), although there have been few detailed studies of its role.
6 Sonochemistry<br />
secCCC01046.1.41.3.1 1.41.3.1 Homogeneous Sonochemistry: Bond Break<strong>in</strong>g and Radical Formation<br />
CCC01046.0105 The chemical effects of ultrasoundon aqueous solutions have been studiedfor many years. The<br />
primary products are H 2 andH 2O 2; there is strong evidence for various high-energy <strong>in</strong>termediates,<br />
<strong>in</strong>clud<strong>in</strong>g HO2, H , OH .[bib17] 17 As one wouldexpect, the sonolysis of water, which<br />
produces both strong reductants and oxidants, is capable of caus<strong>in</strong>g secondary oxidation and<br />
reduction reactions, as often observed by Margulis and co-workers.[bib18] 18 Most recently there<br />
has been strong <strong>in</strong>terest shown <strong>in</strong> the use of ultrasoundfor remediation of low levels of organic<br />
contam<strong>in</strong>ation of water.[bib19] 19 The OH radicals produced from the sonolysis of water are able<br />
to attack essentially all organic compounds (<strong>in</strong>clud<strong>in</strong>g halocarbons, pesticides, and nitroaromatics)<br />
and through a series of reactions oxidize them fully. The ultrasonic irradiation of organic<br />
liquids creates the same k<strong>in</strong>ds of products associated with very-high-temperature pyrolysis.[bib20]<br />
20 The sonochemistry of solutes dissolved <strong>in</strong> organic liquids also rema<strong>in</strong>s largely unexplored. The<br />
sonochemistry of metal carbonyl compounds is an exception. [bib21] 21 Detailedstudies of these<br />
systems ledto important mechanistic understand<strong>in</strong>gs of the nature of sonochemistry. A variety of<br />
unusual reactivity patterns have been observed dur<strong>in</strong>g ultrasonic irradiation, <strong>in</strong>clud<strong>in</strong>g multiple<br />
liganddissociation, novel metal cluster formation, andthe <strong>in</strong>itiation of homogeneous catalysis at<br />
low ambient temperature.[bib21] 21<br />
secCCC01046.1.41.3.2 1.41.3.2 Applications of Sonochemistry to Materials Synthesis<br />
CCC01046.0110 Of special <strong>in</strong>terest is the recent development of sonochemistry as a synthetic tool for the creation<br />
of unusual <strong>in</strong>organic materials.[bib22] 22 As one example, the recent discovery of a simple sonochemical<br />
synthesis of amorphous iron helpedsettle the long-stand<strong>in</strong>g controversy over its magnetic<br />
properties.[bib23 bib24] 23,24 More generally, ultrasoundhas provedextremely useful <strong>in</strong> the<br />
synthesis of a wide range of nanostructured materials, <strong>in</strong>clud<strong>in</strong>g high-surface-area transition<br />
metals, alloys, carbides, oxides, and colloids.[bib22 bib25 bib26 bib27] 22,25–27 Sonochemical decomposition<br />
of volatile organometallic precursors <strong>in</strong> high-boil<strong>in</strong>g solvents produces nanostructured<br />
materials <strong>in</strong> various forms with high catalytic activities. Nanometer colloids; nanoporous highsurface-area<br />
aggregates; nanostructured carbides, sulfides, and oxides; and supported heterogeneous<br />
catalysts can all be preparedby this general route, as shown schematically <strong>in</strong> [figCCC01046.4]<br />
Figure 4. As an example, ultrasonic irradiation of Mo(CO) 6 produces aggregates of nanometersized<br />
clusters of face-centered cubic molybdenum carbide, with high porosity and very large<br />
surface area. The catalytic properties showed that the molybdenum carbide generated by ultrasound<br />
is an active and highly selective dehydrogenation catalyst comparable to commercial<br />
ultraf<strong>in</strong>e plat<strong>in</strong>um powder.[bib25] 25 In related work, Gedanken and co-workers have extended<br />
the sonochemical synthesis of amorphous transition metals to the production of nanostructured<br />
metal oxides simply by sonication <strong>in</strong> the presence of air.[bib26 bib27 bib28] 26–28<br />
<strong>in</strong>organic<br />
support<br />
oxygen<br />
ULTRA-<br />
hydrocarbon<br />
SOUND<br />
stabilizer<br />
Sulfur<br />
source<br />
n = 100–1000<br />
figCCC01046.4 Figure 4 Sonochemical synthesis of nanostructured<strong>in</strong>organic materials.
Sonochemistry 7<br />
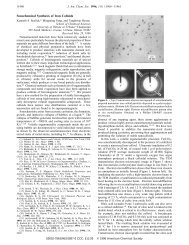
figCCC01046.5 Figure 5 Morphology of conventional andsonochemically preparedMoS2.<br />
CCC01046.0115 The sonochemical synthesis of nanostructured molybdenum sulfide [bib29] 29 provides another<br />
example of the utility of sonochemistry to the production of active catalysts. MoS2 is the<br />
predom<strong>in</strong>ant hydrodesulfurization catalyst heavily used by the petroleum <strong>in</strong>dustry to remove<br />
sulfur from fossil fuels before combustion. The sonochemical synthesis of MoS 2 by the irradiation<br />
of solutions of molybdenum hexacarbonyl generates a most unusual morphology. As shown <strong>in</strong><br />
[figCCC01046.5] Figure 5, conventional MoS2 shows a plate-like morphology typical for such layered<br />
materials, whereas the sonochemical MoS 2 exists as a porous agglomeration of clusters of<br />
spherical particles with an average diameter of 15 nm. Despite the morphological difference<br />
between the sonochemical andconventional MoS2, TEM images of both sulfides show lattice<br />
fr<strong>in</strong>ges with <strong>in</strong>terlayer spac<strong>in</strong>gs of 0.62 0.01 nm. The sonochemically preparedMoS2, however,<br />
shows much greater edge and defect content, as the layers must bend, break, or otherwise distort<br />
to form the outer surface. It is well establishedthat the activity of MoS2 is localizedat the edges<br />
andnot on the flat basal planes. Given the <strong>in</strong>herently higher edge concentrations <strong>in</strong> nanostructuredmaterials,<br />
the catalytic properties of sonochemically preparedMoS 2 shows substantially<br />
<strong>in</strong>creasedactivity for hydrodesulfurization.<br />
CCC01046.0120 Us<strong>in</strong>g a very different route, Grieser and co-workers have sonochemically produced nanocrystall<strong>in</strong>e<br />
CdS colloids. [bib30] 30 In situ-generated hydrogen sulfide from the sonication of 2-mercaptopropionic<br />
acid acted as the sulfid<strong>in</strong>g agent. Spectroscopic studies revealed that sonication<br />
produces CdS particles <strong>in</strong> the quantum dot range (‘‘Q-state’’, 3 nm diameter <strong>in</strong> this study) and<br />
the sonication time and the thiol type determ<strong>in</strong>e the particle size distribution.<br />
secCCC01046.1.41.3.3 1.41.3.3 Heterogeneous Sonochemistry: Reactions of Solids with Liquids<br />
CCC01046.0125 The use of ultrasoundto accelerate chemical reactions <strong>in</strong> heterogeneous systems has become<br />
<strong>in</strong>creas<strong>in</strong>gly widespread. The physical phenomena that are responsible <strong>in</strong>clude the creation of<br />
emulsions at liquid–liquid <strong>in</strong>terfaces, the generation of cavitational erosion and clean<strong>in</strong>g at liquid–<br />
solid <strong>in</strong>terfaces, the production of shockwave damage and deformation of solid surfaces, the<br />
enhancement <strong>in</strong> surface area from fragmentation of friable solids, and the improvement of mass<br />
transport from turbulent mix<strong>in</strong>g andacoustic stream<strong>in</strong>g.<br />
CCC01046.0130 The use of high-<strong>in</strong>tensity ultrasoundto enhance the reactivity of metal powders andsurfaces as<br />
stoichiometric reagents has become an especially rout<strong>in</strong>e synthetic technique for many heterogeneous<br />
organic andorganometallic reactions,[bib1 bib2 bib3] 1–3 particularly those <strong>in</strong>volv<strong>in</strong>g reactive<br />
metals such as Mg, Li, or Zn. This development orig<strong>in</strong>ated from the early work of Renaud<br />
andthe more recent breakthroughs of Luche. [bib3] 3 The effects are quite general andapply to<br />
reactive <strong>in</strong>organic salts andto ma<strong>in</strong> group reagents as well. Less work has been done with<br />
unreactive metals (e.g., V, Nb, Mo, W), but results here are promis<strong>in</strong>g as well. Rate enhancements<br />
of more than tenfold are common, yields are often substantially improved, and by-products
8 Sonochemistry<br />
avoided. A range of synthetically useful examples of heterogeneous sonochemical reactions are<br />
listed<strong>in</strong> [tblCCC01046.1] Table 1.<br />
CCC01046.0135 The mechanism of the sonochemical rate enhancements <strong>in</strong> both stoichiometric andcatalytic<br />
reactions of metals is associated with dramatic changes <strong>in</strong> morphology of both large extended<br />
surfaces and of powders. As discussed earlier, these changes orig<strong>in</strong>ate from microjet impact on<br />
large surfaces andhigh-velocity <strong>in</strong>terparticle collisions <strong>in</strong> slurries. Surface composition studies by<br />
Auger electron spectroscopy andsputteredneutral mass spectrometry reveal that ultrasonic<br />
irradiation effectively removes surface oxide and other contam<strong>in</strong>at<strong>in</strong>g coat<strong>in</strong>gs.[bib9] 9 The removal<br />
of such passivat<strong>in</strong>g coat<strong>in</strong>gs can dramatically improve reaction rates. The reactivity of clean metal<br />
tblCCC01046.1 Table 1 Some representative examples of heterogeneous sonochemistry.<br />
Heterogeneous reagent Reactant Products<br />
Compounds of metals<br />
LiAlH4 Ar-X ArH<br />
LiAlH4 R3M-X (X¼Cl, NR2,OR) R3M-H (M¼Si,Ge,Sn)<br />
Al 2O 3 C 6H 5CH 2Br þ KCN C 6H 5CH 2CN<br />
KMnO 4 RR 0 HCOH RR 0 CO<br />
CrO2Cl2 RR 0 HCOH RR 0 CO<br />
HBR2 R 0 2C¼CR 0 2 HR 0 2C-CR 0 2(BR2)<br />
MS 2,V 2O 5 NR 3, py, Cp 2Co Intercalates<br />
Hg<br />
Mg<br />
Li<br />
Zn<br />
Transition metals<br />
(HR2BrC)2CO þ R 0 CO2H (HR2C)CO(C(O2CR 0 )R2)<br />
(HR 2BrC) 2CO þ R 0 OH (HR 2C)CO(C(OR 0 )R 2)<br />
R-Br R-MgBr<br />
R2C¼CHCH2Cl þ Mg/C14H14 R2C¼CHCH2MgCl R-Br (R ¼ Pr, n-Bu, Ph) R-Li<br />
R-Br þ R 0 R 00 CO RR 0 R 00 COH<br />
R-Br þ (H3C)2NCHO RCHO<br />
R3M-Cl (M¼C,Si,Sn) R3MMR3<br />
R 2SiCl 2 (R ¼ arenes) cyclo-(R 2Si) 3<br />
R2MCl2 þ Na þ Se (M¼Si,Sn) (R2MSe)3<br />
CF3I þ RR0C¼O RR0C(OH)CF3 CnF2nþ1CO2H<br />
CnF2nþ1I þ CO2<br />
RR0C¼O þ BrCH2CO2R00 RR0C(OH)CH2CO2R00 PhBr þ RCOCH¼CHR0 þ Ni(acac) 2 RCOCH2CHR0Ph (R=H,alkyl)<br />
RR 0 C¼O þ R 00 2C¼CHCH 2Br RR 0 (HO)CCR 00 2CH¼CH 2<br />
MCl5 þ Na þ CO (M¼V,Nb,Ta) M(CO) 6<br />
MCl6 þ Na þ CO (M¼Cr,Mo,W) M2(CO)10 2<br />
MnCl3 þ Na þ CO Mn(CO)5<br />
FeCl3 þ Na þ CO Fe(CO)4 2 þ Fe2(CO)8 2<br />
Fe2(CO) 9 þ alkenylepoxide Fe(CO) 3( -allyllactone)<br />
Fe2(CO)9 þ RHC¼CR0-CH ¼CHR0 Fe(CO)3( 4 -diene)<br />
NiCl2 þ Na þ CO Ni6(CO)12 2<br />
NiCl2 þ Na þ bipy þ COD/CDT Ni(bipy)(COD/CDT)<br />
Co(acac) 3 þ C5H6 þ COD þ Mg/C14H10 Co(Cp)(COD)<br />
RuCl3 þ Li þ 1,5-cyclooctadiene ( 6 -1,3,5-COT)( 4 -1,5-COD)Ru(0)<br />
[Fe(C5R5)(CO)2]2 þ K þ Ph3C þ<br />
Fe(C5R5)(CO)2(C2H4) þ<br />
[M(C5R5)(CO) n] 2 þ K(M¼Fe,Ru,Mo) [M(C5R5)(CO) n]<br />
Pd þ CH2¼CHCH2X [( 3 -H2C-CH-CH2)Pd(X)]2<br />
La þ NH4SCN <strong>in</strong> HMPA La(NCS)3 4HMPA<br />
Cu þ o-C6H4(NO2)I o-(O2N)H4C6–C6H4(NO2)
surfaces also appears to be responsible for the greater tendency for heterogeneous sonochemical<br />
reactions to <strong>in</strong>volve s<strong>in</strong>gle electron transfer rather than acid-base chemistry.[bib31] 31<br />
CCC01046.0140 Significant applications of sonochemistry to heterogeneous catalysis have also been noted. [bib32]<br />
32 Among the more impressive results are hydrogenations and hydrosilations by Ni powder, Raney<br />
Ni, andPdor Pt on carbon. For example, the hydrogenation of alkenes by Ni powder is enormously<br />
enhanced(>10 5 -fold) by ultrasonic irradiation.[bib10] 10 This dramatic <strong>in</strong>crease <strong>in</strong> catalytic activity is<br />
due to the formation of uncontam<strong>in</strong>ated metal surfaces from <strong>in</strong>terparticle collisions caused by<br />
cavitation-<strong>in</strong>duced shockwaves.<br />
SECCCC01046.1.41.4 1.41.4 CONCLUSIONS<br />
CCC01046.0145 S<strong>in</strong>ce the early 1980 s, sonochemistry has become a well-def<strong>in</strong>ed technique for both mechanistic<br />
and synthetic studies. The general details of the process of acoustic cavitation and the high-energy<br />
chemistry generated dur<strong>in</strong>g such bubble collapse are now reasonably well understood. The major<br />
challenges that face a wider application of this technology <strong>in</strong>clude issues of scale-up and energy<br />
efficiency. While laboratory equipment for sonochemical reactors are readily available commercially,<br />
larger-scale apparatus is only now becom<strong>in</strong>g commercially available. Perhaps more importantly,<br />
sonochemistry shares with photochemistry an energy <strong>in</strong>efficiency that rema<strong>in</strong>s<br />
problematic: while the production of ultrasound from electrical power can be extremely efficient,<br />
the coupl<strong>in</strong>g of ultrasound<strong>in</strong>to chemically useful cavitation events rema<strong>in</strong>s a low-yieldevent. As<br />
we beg<strong>in</strong> to understand the physics of cavitation clouds and dense multiphase acoustics, more<br />
effective means of <strong>in</strong>duc<strong>in</strong>g cavitation <strong>in</strong> liquids may alleviate this current limitation.<br />
1.41.5 REFERENCES<br />
Sonochemistry 9<br />
[bib1] 1. Crum, L. A.; Mason, T. J.; Reisse, J.; Suslick, K.S., Eds. Sonochemistry and Sonolum<strong>in</strong>escence, NATO ASI Series<br />
C, v. 524; Kluwer: Dordrecht, The Netherlands, 1999.<br />
[bib2] 2. Mason, T. J.; Lorimer, J. P. Sonochemistry: Theory, Applications and Uses of Ultrasound <strong>in</strong> <strong>Chemistry</strong> 1988, Ellis<br />
Horword: Chichester, UK.<br />
[bib3] 3. Luche, J.-L. Synthetic Organic Sonochemistry 1998, NewYork: Plenum.<br />
[bib4] 4. Suslick, K. S.; Cl<strong>in</strong>e, Jr., R. E.; Hammerton, D. A. J.Am.Chem.Soc.1986, 108, 5641–5642.<br />
[bib5] 5. Fl<strong>in</strong>t, E. B.; Suslick, K. S. Science 1991, 253, 1397–1399.<br />
[bib6] 6. McNamara III, W. B.; Didenko, Y.; Suslick, K. S. Nature 1999, 401, 772–775.<br />
[bib7] 7. Leighton, T. G. The Acoustic Bubble 1994, Academic Press: London.<br />
[bib8] 8. Suslick, K. S.; Doktycz, S. J. Science 1990, 247, 1067–1069.<br />
[bib9] 9. Suslick, K. S.; Doktycz, S. J. Adv.Sonochem.1990, 1, 197–230.<br />
[bib10]10. Suslick, K. S.; Casadonte, D. J. J.Am.Chem.Soc.1987, 109, 3459–3461.<br />
[bib11]11. Crum, L. A. Phys.Today 1994, 47, 22–29.<br />
[bib12]12. S. J. Putterman, Sci.Am.1995, (Feb.), 46–52.<br />
[bib13]13. LordRayleigh, Philos.Mag.Ser.6 1917, 34, 94–98.<br />
[bib14]14. Richards, W. T.; Loomis, A. L. J.Am.Chem.Soc.1927, 49, 3086–3100.<br />
[bib15]15. Frenzel, H.; Schultes, H. Z.Phys.Chem.1934, 27b, 421–424.<br />
[bib16]16. Suslick, K. S.; Crum, L. A. Sonochemistry andSonolum<strong>in</strong>escence. In Encyclopedia of Acoustics; Crocker, M. J.,<br />
Ed., Wiley-Interscience: New York, 1997; Vol. 1, pp 271–282.<br />
[bib17]17. Riesz, P. Adv.Sonochem.1991, 2, 23–64.<br />
[bib18]18. Margulis, M. A.; Maximenko, N. A. Adv.Sonochem.1991, 2, 253–292.<br />
[bib19]19. Destaillats, H.; Lesko, T. M.; Knowlton, M.; Wallace, H.; Hoffmann, M. R. Ind.Eng.Chem.Res.2001, 40,<br />
3855–3860.<br />
[bib20]20. Suslick, K. S.; Gawienowski, J. W.; Schubert, P. F.; Wang, H. H. J.Phys.Chem.1983, 87, 2299–2301.<br />
[bib21]21. Suslick, K. S. Adv.Organomet.Chem.1986, 25, 73–119.<br />
[bib22]22. Suslick, K. S.; Price, G. Annu.Rev.Mater.Sci.1999, 29, 295–326.<br />
[bib23]23. Suslick, K. S.; Choe, S. B.; Cichowlas, A. A.; Gr<strong>in</strong>staff, M. W. Nature 1991, 353, 414–416.<br />
[bib24]24. Gr<strong>in</strong>staff, M. W.; Salamon, M. B.; Suslick, K. S. Phys.Rev.B 1993, 48, 269–273.<br />
[bib25]25. Hyeon, T.; Fang, M.; Suslick, K. S. J.Am.Chem.Soc.1996, 118, 5492–5493.<br />
[bib26]26. Cao, X.; Prozorov, R.; Koltyp<strong>in</strong>, Y.; Kataby, G.; Felner, I.; Gedanken, A. J.Mater.Res.1997, 122, 402–406.<br />
[bib27]27. Shafi, K. V. P. M.; Koltyp<strong>in</strong>, Y.; Gedanken, A.; Prozorov, R.; Balogh, J.; Lendvai, J.; Felner, I. J.Phys.Chem.<br />
B. 1997, 101, 6409–6414.<br />
[bib28]28. Kataby, G.; Prozorov, T; Koltyp<strong>in</strong>, Y.; Cohen, H.; Sukenik, C. N.; Ulman, A.; Gedanken, A. Langmuir 1997, 13,<br />
6151–6158.<br />
[bib29]29. Mdleleni, M. M.; Hyeon, T.; Suslick, K. S. J.Am.Chem.Soc.1998, 120, 6189–6190.<br />
[bib30]30. Sostaric, J. Z.; Carusohobson, R. A.; Mulvaney, P.; Grieser, F. J.Chem.Soc.Faraday Trans.1997, 93,<br />
1791–1795.<br />
[bib31]31. Luche, J.-L. Ultrasonics Sonochem. 1994, 1, S111.<br />
[bib32]32. Suslick, K. S. In Handbook of Heterogeneous Catalysis; Ertl, G.; Knoz<strong>in</strong>ger, H.; Weitkamp, J., Eds.; Wiley-VCH:<br />
We<strong>in</strong>heim, 1997; Vol. 3, pp 1350–1357.