Program - International Chinese Statistical Association
Program - International Chinese Statistical Association
Program - International Chinese Statistical Association
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
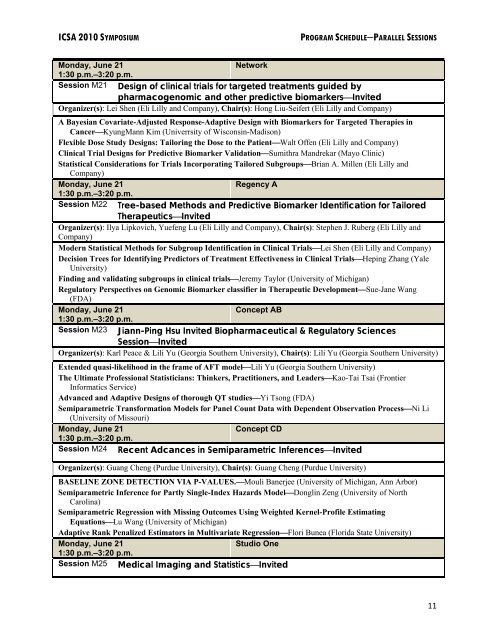
ICSA 2010 SYMPOSIUM PROGRAM SCHEDULE−PARALLEL SESSIONS<br />
Monday, June 21<br />
Network<br />
1:30 p.m.–3:20 p.m.<br />
Session M21 Design of clinical trials for targeted treatments guided by<br />
pharmacogenomic and other predictive biomarkers⎯Invited<br />
Organizer(s): Lei Shen (Eli Lilly and Company), Chair(s): Hong Liu-Seifert (Eli Lilly and Company)<br />
A Bayesian Covariate-Adjusted Response-Adaptive Design with Biomarkers for Targeted Therapies in<br />
Cancer⎯KyungMann Kim (Univerrsity of Wisconsin-Madison)<br />
Flexible Dose Study Designs: Tailoring the Dose to the Patient⎯Walt Offen (Eli Lilly and Company)<br />
Clinical Trial Designs for Predictive Biomarker Validation⎯Sumithra Mandrekar (Mayo Clinic)<br />
<strong>Statistical</strong> Considerations for Trials Incorporating Tailored Subgroups⎯Brian A. Millen (Eli Lilly and<br />
Company)<br />
Monday, June 21<br />
Regency A<br />
1:30 p.m.–3:20 p.m.<br />
Session M22 Tree-based Methods and Predictive Biomarker Identification for Tailored<br />
Therapeutics⎯Invited<br />
Organizer(s): Ilya Lipkovich, Yuefeng Lu (Eli Lilly and Company), Chair(s): Stephen J. Ruberg (Eli Lilly and<br />
Company)<br />
Modern <strong>Statistical</strong> Methods for Subgroup Identification in Clinical Trials⎯Lei Shen (Eli Lilly and Company)<br />
Decision Trees for Identifying Predictors of Treatment Effectiveness in Clinical Trials⎯Heping Zhang (Yale<br />
University)<br />
Finding and validating subgroups in clinical trials⎯Jeremy Taylor (University of Michigan)<br />
Regulatory Perspectives on Genomic Biomarker classifier in Therapeutic Development⎯Sue-Jane Wang<br />
(FDA)<br />
Monday, June 21<br />
Concept AB<br />
1:30 p.m.–3:20 p.m.<br />
Session M23 Jiann-Ping Hsu Invited Biopharmaceutical & Regulatory Sciences<br />
Session⎯Invited<br />
Organizer(s): Karl Peace & Lili Yu (Georgia Southern University), Chair(s): Lili Yu (Georgia Southern University)<br />
Extended quasi-likelihood in the frame of AFT model⎯Lili Yu (Georgia Southern University)<br />
The Ultimate Professional Statisticians: Thinkers, Practitioners, and Leaders⎯Kao-Tai Tsai (Frontier<br />
Informatics Service)<br />
Advanced and Adaptive Designs of thorough QT studies⎯Yi Tsong (FDA)<br />
Semiparametric Transformation Models for Panel Count Data with Dependent Observation Process⎯Ni Li<br />
(University of Missouri)<br />
Monday, June 21<br />
Concept CD<br />
1:30 p.m.–3:20 p.m.<br />
Session M24 Recent Adcances in Semiparametric Inferences⎯Invited<br />
Organizer(s): Guang Cheng (Purdue University), Chair(s): Guang Cheng (Purdue University)<br />
BASELINE ZONE DETECTION VIA P-VALUES.⎯Mouli Banerjee (University of Michigan, Ann Arbor)<br />
Semiparametric Inference for Partly Single-Index Hazards Model⎯Donglin Zeng (University of North<br />
Carolina)<br />
Semiparametric Regression with Missing Outcomes Using Weighted Kernel-Profile Estimating<br />
Equations⎯Lu Wang (University of Michigan)<br />
Adaptive Rank Penalized Estimators in Multivariate Regression⎯Flori Bunea (Florida State University)<br />
Monday, June 21<br />
Studio One<br />
1:30 p.m.–3:20 p.m.<br />
Session M25 Medical Imaging and Statistics⎯Invited<br />
11