Program - International Chinese Statistical Association
Program - International Chinese Statistical Association
Program - International Chinese Statistical Association
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
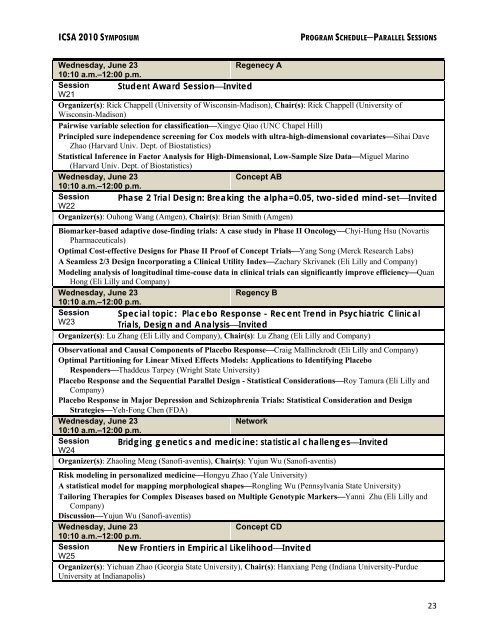
ICSA 2010 SYMPOSIUM PROGRAM SCHEDULE−PARALLEL SESSIONS<br />
Wednesday, June 23<br />
Regenecy A<br />
10:10 a.m.–12:00 p.m.<br />
Session Student Award Session⎯Invited<br />
W21<br />
Organizer(s): Rick Chappell (University of Wisconsin-Madison), Chair(s): Rick Chappell (University of<br />
Wisconsin-Madison)<br />
Pairwise variable selection for classification⎯Xingye Qiao (UNC Chapel Hill)<br />
Principled sure independence screening for Cox models with ultra-high-dimensional covariates⎯Sihai Dave<br />
Zhao (Harvard Univ. Dept. of Biostatistics)<br />
<strong>Statistical</strong> Inference in Factor Analysis for High-Dimensional, Low-Sample Size Data⎯Miguel Marino<br />
(Harvard Univ. Dept. of Biostatistics)<br />
Wednesday, June 23<br />
Concept AB<br />
10:10 a.m.–12:00 p.m.<br />
Session Phase 2 Trial Design: Breaking the alpha=0.05, two-sided mind-set⎯Invited<br />
W22<br />
Organizer(s): Ouhong Wang (Amgen), Chair(s): Brian Smith (Amgen)<br />
Biomarker-based adaptive dose-finding trials: A case study in Phase II Oncology⎯Chyi-Hung Hsu (Novartis<br />
Pharmaceuticals)<br />
Optimal Cost-effective Designs for Phase II Proof of Concept Trials⎯Yang Song (Merck Research Labs)<br />
A Seamless 2/3 Design Incorporating a Clinical Utility Index⎯Zachary Skrivanek (Eli Lilly and Company)<br />
Modeling analysis of longitudinal time-couse data in clinical trials can significantly improve efficiency⎯Quan<br />
Hong (Eli Lilly and Company)<br />
Wednesday, June 23<br />
Regency B<br />
10:10 a.m.–12:00 p.m.<br />
Session<br />
W23<br />
Special topic: Placebo Response - Recent Trend in Psychiatric Clinical<br />
Trials, Design and Analysis⎯Invited<br />
Organizer(s): Lu Zhang (Eli Lilly and Company), Chair(s): Lu Zhang (Eli Lilly and Company)<br />
Observational and Causal Components of Placebo Response⎯Craig Mallinckrodt (Eli Lilly and Company)<br />
Optimal Partitioning for Linear Mixed Effects Models: Applications to Identifying Placebo<br />
Responders⎯Thaddeus Tarpey (Wright State University)<br />
Placebo Response and the Sequential Parallel Design - <strong>Statistical</strong> Considerations⎯Roy Tamura (Eli Lilly and<br />
Company)<br />
Placebo Response in Major Depression and Schizophrenia Trials: <strong>Statistical</strong> Consideration and Design<br />
Strategies⎯Yeh-Fong Chen (FDA)<br />
Wednesday, June 23<br />
Network<br />
10:10 a.m.–12:00 p.m.<br />
Session Bridging genetics and medicine: statistical challenges⎯Invited<br />
W24<br />
Organizer(s): Zhaoling Meng (Sanofi-aventis), Chair(s): Yujun Wu (Sanofi-aventis)<br />
Risk modeling in personalized medicine⎯Hongyu Zhao (Yale University)<br />
A statistical model for mapping morphological shapes⎯Rongling Wu (Pennsylvania State University)<br />
Tailoring Therapies for Complex Diseases based on Multiple Genotypic Markers⎯Yanni Zhu (Eli Lilly and<br />
Company)<br />
Discussion⎯Yujun Wu (Sanofi-aventis)<br />
Wednesday, June 23<br />
Concept CD<br />
10:10 a.m.–12:00 p.m.<br />
Session New Frontiers in Empirical Likelihood⎯Invited<br />
W25<br />
Organizer(s): Yichuan Zhao (Georgia State University), Chair(s): Hanxiang Peng (Indiana University-Purdue<br />
University at Indianapolis)<br />
23