Microstructural characterization of Ruddlesden-Popper ... - cnpem
Microstructural characterization of Ruddlesden-Popper ... - cnpem
Microstructural characterization of Ruddlesden-Popper ... - cnpem
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Microstructural</strong> <strong>characterization</strong> <strong>of</strong> <strong>Ruddlesden</strong>-<strong>Popper</strong> nickelates for use in catalysis<br />
Pimentel, P. M., 1 Oliveira, R. M. P. B., 1 Oliveira, F. S., 1 Melo, D. M. A., 1 and Melo,M.A.F. 1<br />
INTRODUCTION<br />
Several catalysts containing noble metals supported (Rh,<br />
Pt, Ru, Pd) have shown high conversion and high selectivity<br />
for production <strong>of</strong> syngas (mixture <strong>of</strong> CO and H2) [1, 2].<br />
However, the high cost <strong>of</strong> noble metals has motivated the development<br />
<strong>of</strong> nickel catalysts for industrial use and one <strong>of</strong><br />
the challenges is to make it more resistant to carbon deposition.<br />
The most widely used commercial catalyst is nickel<br />
supported on alumina, with or without promoters. In recent<br />
years, much attention has been given to the study <strong>of</strong> oxide<br />
systems with perovskite structure and its application as catalysts,<br />
mainly oxides, which presents the Ruddelsden-<strong>Popper</strong><br />
(RP) phase. The RP phases with general formula <strong>of</strong> A2BO4<br />
consist <strong>of</strong> n ABO3 perovskite blocks, separated by a rock saltlike<br />
layer <strong>of</strong> composition AO. These materials are usually obtained<br />
through routes which make use <strong>of</strong> high pressures and<br />
high temperatures [3]. The aim <strong>of</strong> this work was to characterize<br />
the microstructure <strong>of</strong> the RP phases <strong>of</strong> nickelates in order<br />
to the understanding <strong>of</strong> their catalytic properties.<br />
EXPERIMENT<br />
The systems were synthesized by using gelatin powder<br />
(GELITA R○ ) as organic precursor and metallic nitrates as<br />
starting materials. Gelatin was added to a becker containing<br />
deionized water under stirring for 30 minutes at 50 o C.<br />
M(NO3)2.6H2O (M = Ni, Nd or Sm, Sr) were added to the<br />
solution at 70 o C for some minutes. The temperature was<br />
slowly increased to 90 o C and the solution was stirred on<br />
a hot plate until a gel formed. The gel was then calcined<br />
at 350 o C for 2 hours. This resulted in a precursor powder,<br />
which was calcined at 900 o C for 4 hours. The system containing<br />
neodymium was calcinated at 900 o C for 6 hours to<br />
evaluate the behavior <strong>of</strong> the structure. The X-ray patterns<br />
were acquired from samples calcined at different temperatures.<br />
Measurements were recorded on a Shimadzu diffractometer<br />
model XRD-6000 with monochromatic radiation <strong>of</strong><br />
CuK α (λ = 1.5406 Å). The crystallites size was obtained<br />
from the Scherrer equation. The FEG-SEM images <strong>of</strong> the<br />
powders were examined in a JEOL JSM 6330F microscopy<br />
at the LNLS - National Synchrotron Light Source (Campinas-<br />
Brazil).<br />
RESULTS AND DISCUSSION<br />
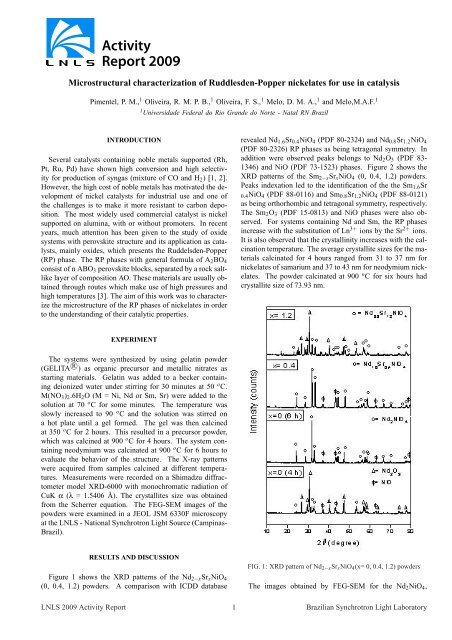
Figure 1 shows the XRD patterns <strong>of</strong> the Nd2−xSrxNiO4<br />
(0, 0.4, 1.2) powders. A comparison with ICDD database<br />
1 Universidade Federal do Rio Grande do Norte - Natal RN Brazil<br />
revealed Nd1.6Sr0.4NiO4 (PDF 80-2324) and Nd0.8Sr1.2NiO4<br />
(PDF 80-2326) RP phases as being tetragonal symmetry. In<br />
addition were observed peaks belongs to Nd2O3 (PDF 83-<br />
1346) and NiO (PDF 73-1523) phases. Figure 2 shows the<br />
XRD patterns <strong>of</strong> the Sm2−xSrxNiO4 (0, 0.4, 1.2) powders.<br />
Peaks indexation led to the identification <strong>of</strong> the the Sm1.6Sr<br />
0.4NiO4 (PDF 88-0116) and Sm0.8Sr1.2NiO4 (PDF 88-0121)<br />
as being orthorhombic and tetragonal symmetry, respectively.<br />
The Sm2O3 (PDF 15-0813) and NiO phases were also observed.<br />
For systems containing Nd and Sm, the RP phases<br />
increase with the substitution <strong>of</strong> Ln 3+ ions by the Sr 2+ ions.<br />
It is also observed that the crystallinity increases with the calcination<br />
temperature. The average crystallite sizes for the materials<br />
calcinated for 4 hours ranged from 31 to 37 nm for<br />
nickelates <strong>of</strong> samarium and 37 to 43 nm for neodymium nickelates.<br />
The powder calcinated at 900 o C for six hours had<br />
crystallite size <strong>of</strong> 73.93 nm.<br />
FIG. 1: XRD pattern <strong>of</strong> Nd2−xSrxNiO4(x= 0, 0.4, 1.2) powders<br />
The images obtained by FEG-SEM for the Nd2NiO4,<br />
LNLS 2009 Activity Report 1 Brazilian Synchrotron Light Laboratory
FIG. 2: XRD pattern <strong>of</strong> Sm2−xSrxNiO4 (x= 0, 0.4, 1.2) powders<br />
Nd0.8Sr1.2NiO4 and Sm0.8Sr1.2NiO4 samples are shown in<br />
Fig. 3 and 4, respectively. As can be seen, the particles<br />
have a round shape, uniform distribution and do not show noticeable<br />
agglomeration. The porous surface <strong>of</strong> the material is<br />
caused by the evolution <strong>of</strong> high gas content during synthesis<br />
and powder production. The gelatin provides the system with<br />
a large amount <strong>of</strong> organic matter, which during calcination is<br />
removed and favors the appearance <strong>of</strong> pores in the material.<br />
The materials calcinated for 4 h showed nanometric particle<br />
size, but in the material calcined for 6 h, there was a significant<br />
increase in the particle size.<br />
FIG. 3: SEM-FEG images <strong>of</strong> a) Nd2NiO4 calcinated for 6 h and b)<br />
Nd1.6Sr0.4NiO4 calcinated for 4 h<br />
FIG. 4: SEM-FEG images <strong>of</strong> Sm0.8Sr 1.2NiO4 calcinated for 4 h<br />
CONCLUSION<br />
In this work, perovskite type RP was obtained successfully<br />
through a simple and fast method and makes use <strong>of</strong> lower temperatures<br />
and pressures compared to other work in literature.<br />
In addition, gelatin is a low-cost and non-toxic organic precursor,<br />
making it a promising alternative to the usual synthesis<br />
methods. Powders produced by using this method were<br />
nanometric, homogeneous and porous. These characteristics<br />
are important for technological application such as catalysts.<br />
ACKNOWLEDGEMENTS<br />
The authors are grateful to CNPq for the financial support,<br />
GELITA R○ for supplying the gelatin and Brazilian Synchrotron<br />
Light Laboratory (LNLS) for SEM-FEG images<br />
(SEM-FEG-9042/2009).<br />
[1] E. Boehm, J.M. Bassat, P. Dordor, F. Mauvy, J.C. Grenier, P.<br />
Stevens, Solid State Ionics 176, 2717 (2005)<br />
[2] G.Wu, J.J. Neumeier, M.F. Hundley, Phys. Rev. B 63, 1 (2001)<br />
[3] M. Zinkevich , N. Solak, H. Nitsche, M. Ahrens, F. Aldinger.<br />
Journal <strong>of</strong> Alloys and Compounds 438, 92 (2007)<br />
LNLS 2009 Activity Report 2 Brazilian Synchrotron Light Laboratory