Crystal Growth Mineral Formation & Stability Gibbs Free Energy ...
Crystal Growth Mineral Formation & Stability Gibbs Free Energy ...
Crystal Growth Mineral Formation & Stability Gibbs Free Energy ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
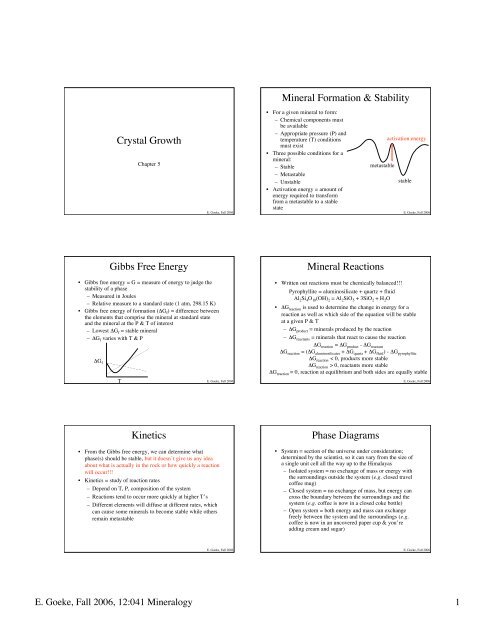
<strong>Crystal</strong> <strong>Growth</strong><br />
Chapter 5<br />
<strong>Gibbs</strong> <strong>Free</strong> <strong>Energy</strong><br />
E. Goeke, Fall 2006<br />
• <strong>Gibbs</strong> free energy = G = measure of energy to judge the<br />
stability of a phase<br />
– Measured in Joules<br />
– Relative measure to a standard state (1 atm, 298.15 K)<br />
• <strong>Gibbs</strong> free energy of formation (ΔG f) = difference between<br />
the elements that comprise the mineral at standard state<br />
and the mineral at the P & T of interest<br />
– Lowest ΔG f = stable mineral<br />
– ΔG f varies with T & P<br />
ΔG f<br />
T<br />
Kinetics<br />
E. Goeke, Fall 2006<br />
• From the <strong>Gibbs</strong> free energy, we can determine what<br />
phase(s) should be stable, but it doesn’t give us any idea<br />
about what is actually in the rock or how quickly a reaction<br />
will occur!!!<br />
• Kinetics = study of reaction rates<br />
– Depend on T, P, composition of the system<br />
– Reactions tend to occur more quickly at higher T’s<br />
– Different elements will diffuse at different rates, which<br />
can cause some minerals to become stable while others<br />
remain metastable<br />
E. Goeke, Fall 2006<br />
<strong>Mineral</strong> <strong>Formation</strong> & <strong>Stability</strong><br />
• For a given mineral to form:<br />
– Chemical components must<br />
be available<br />
– Appropriate pressure (P) and<br />
temperature (T) conditions<br />
must exist<br />
• Three possible conditions for a<br />
mineral:<br />
– Stable<br />
– Metastable<br />
– Unstable<br />
• Activation energy = amount of<br />
energy required to transform<br />
from a metastable to a stable<br />
state<br />
metastable<br />
<strong>Mineral</strong> Reactions<br />
• Written out reactions must be chemically balanced!!!<br />
Pyrophyllite = aluminosilicate + quartz + fluid<br />
Al 2Si 4O 10(OH) 2 = Al 2SiO 5 + 3SiO 2 + H 2O<br />
activation energy<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 1<br />
stable<br />
• ΔGreaction is used to determine the change in energy for a<br />
reaction as well as which side of the equation will be stable<br />
at a given P & T<br />
– ΔGproduct = minerals produced by the reaction<br />
– ΔGreactants = minerals that react to cause the reaction<br />
ΔGreaction = ΔGproduct - ΔGreactant ΔGreaction = (ΔGaluminosilicates + ΔGquartz + ΔGfluid) - ΔGpyrophyllite ΔGreaction < 0, products more stable<br />
ΔGreaction > 0, reactants more stable<br />
ΔGreaction = 0, reaction at equilibrium and both sides are equally stable<br />
Phase Diagrams<br />
• System = section of the universe under consideration;<br />
determined by the scientist, so it can vary from the size of<br />
a single unit cell all the way up to the Himalayas<br />
– Isolated system = no exchange of mass or energy with<br />
the surroundings outside the system (e.g. closed travel<br />
coffee mug)<br />
– Closed system = no exchange of mass, but energy can<br />
cross the boundary between the surroundings and the<br />
system (e.g. coffee is now in a closed coke bottle)<br />
– Open system = both energy and mass can exchange<br />
freely between the system and the surroundings (e.g.<br />
coffee is now in an uncovered paper cup & you’re<br />
adding cream and sugar)<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006
• Phase = physically separable portion of the system that is<br />
chemically and physically distinct from everything else in<br />
the system (e.g. one phase is present when sugar & milk is<br />
dissolved in the coffee, but two phases exist when the<br />
sugar is sitting on the bottom of the cup instead of<br />
dissolved in the coffee)<br />
• Components = each phase is composed of 1+ components;<br />
chosen by scientist, but you try to choose as few<br />
components as possible that describe your entire system<br />
http://www.tulane.edu/~sanelson/eens211/mineral_stability.htm<br />
1<br />
3<br />
http://csmres.jmu.edu/geollab/Fichter/IgnRx/BinryEu.html<br />
2<br />
A<br />
B<br />
AB<br />
BC<br />
BCA<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
Phase Rule<br />
• Phase rule = used to determine how many variables &<br />
equations are required to describe a system that’s in<br />
equilibrium<br />
• Variables:<br />
– Number of chemical components<br />
– Extensive variables (pressure & temperature)<br />
• F = C + 2 - P<br />
– F = degrees of freedom or variance of the system<br />
– C = number of components (as defined on the last slide)<br />
– P = number of phases in equilibrium<br />
– 2 comes from P & T<br />
2 Component Eutectic Systems<br />
http://csmres.jmu.edu/geollab/Fichter/IgnRx/BinryEu.html<br />
http://csmres.jmu.edu/geollab/Fichter/IgnRx/BinryEu.html<br />
E. Goeke, Fall 2006<br />
• Liquidus = line<br />
that separates all<br />
melt and melt +<br />
xtal<br />
• Solidus = line that<br />
separates melt +<br />
xtal and all xtal<br />
• Eutectic = point at<br />
which melt + A +<br />
B exists<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 2
EQ Melting<br />
E. Goeke, Fall 2006<br />
Lever rule = how<br />
much L vs. xtal<br />
is present<br />
%xtals of A =<br />
b/(a+b)(100)<br />
%liquid =<br />
a/(a+b)(100)<br />
E. Goeke, Fall 2006<br />
Fractional Melting<br />
http://csmres.jmu.edu/geollab/Fichter/IgnRx/BinryEu.html<br />
Binary Complete Solid Solution<br />
http://csmres.jmu.edu/geollab/Fichter/IgnRx/SolidSol.html<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
We can also use<br />
the Lever rule<br />
for binary solid<br />
solution<br />
diagrams<br />
% solid =<br />
[x/(x+y)][100]<br />
%liquid =<br />
[y/(x+y)][100]<br />
http://csmres.jmu.edu/geollab/Fichter/IgnRx/SolidSol.html E. Goeke, Fall 2006<br />
http://www.tulane.edu/~sanelson/eens211/2compphasdiag.html E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 3
http://www.brocku.ca/earthsciences/people/gfinn/petrology/ab-an2.gif<br />
Nucleation<br />
EQ xtalization -><br />
final xtal comp =<br />
starting L comp<br />
E. Goeke, Fall 2006<br />
• For minerals to nucleate, the new cluster of atoms/ions<br />
must have a lower ΔG then the other phases in the system<br />
(e.g. melt, aqueous solution, other minerals)<br />
• Whether or not a mineral will nucleate will depend on P, T,<br />
and chemical composition of the system<br />
• Size of nucleus will determine whether the new mineral<br />
will grow in size or dissolve<br />
– Greater surface energy = less stable<br />
– Small xtals have a high surface area to volume ratio<br />
S.A. = 6 * 2 * side length<br />
V = (side length) 3<br />
http://www.tulane.edu/~sanelson/eens212/metamorphreact.htm<br />
S.A. = 6 * 2 * 5<br />
V = 5 3<br />
S.A./V = 60/125<br />
S.A. = 6 * 2 * 1<br />
V = 1 3<br />
S.A./V = 12/1<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
http://www.brocku.ca/earthsciences/people/gfinn/petrology/ab-an3.gif<br />
– T d = melt has an equal<br />
ΔG to the xtal, so if<br />
nuclei form, the high<br />
surface energy causes the<br />
xtals to simply dissolve<br />
back into the melt<br />
– T a = xtals have a slightly<br />
lower ΔG than the melt,<br />
but nuclei must be above<br />
a critical size in order to<br />
avoid being resorbed;<br />
only a small number of<br />
nuclei are present & grow<br />
– T b = xtals have a<br />
significantly lower ΔG<br />
than the melt, so many<br />
nuclei form & grow<br />
fractional<br />
xtalization -><br />
final xtal comp ≠<br />
starting L comp<br />
E. Goeke, Fall 2006<br />
Winter, 2001, An Introduction to Igneous & Metamorphic Geology<br />
Heterogeneous Nucleation<br />
• Heterogeneous nucleation occurs by a new xtal taking<br />
advantage of a pre-existing surface or flaw to nucleate<br />
– Largely eliminates the need for seed nuclei<br />
– Reduces surface energy problems<br />
• Can occur on:<br />
– Specific crystallographic faces of a pre-existing xtal =<br />
epitaxial nucleation<br />
– On a structural defect such as as grain boundary or<br />
another imperfection, where the surface energy was<br />
higher for the original xtal but the new nucleation<br />
lowers the surface energy<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 4<br />
T d<br />
E. Goeke, Fall 2006
<strong>Crystal</strong> <strong>Growth</strong><br />
• Still have to worry about surface<br />
energy<br />
– Less when new growth is<br />
along a pre-existing row or at<br />
flaw in the xtal<br />
– Screw dislocations cause<br />
growth that continually has an<br />
edge available<br />
– New growth will try and<br />
reduce the surface energy of<br />
the pre-existing xtal<br />
Zoned <strong>Crystal</strong>s<br />
• As a xtal grows, it changes the composition of the<br />
melt/rock that its growing from<br />
• Some minerals can have a variation in their composition<br />
(e.g. garnet (Fe,Mg,Ca,Mn) 3(Al,Fe) 2Si 3O 12, olivine<br />
(Fe,Mg) 2SiO 4, plagioclase (Na,Ca)Al(Al,Si)Si 2O 8), which<br />
means that the xtal could change compositions as it grows<br />
• Changes in P, T, and bulk composition can all cause<br />
zoning<br />
E. Goeke, Fall 2006<br />
Rate of <strong>Growth</strong><br />
• Xtals will grow fastest usually along<br />
longer dimensions of the unit cell (e.g.<br />
halite will grow fastest along the<br />
{111} faces & slower along the {100}<br />
faces)<br />
• Slow growing faces tend to be more<br />
prominent<br />
– Faces with higher surface energy<br />
will grow faster<br />
– Xtal growth tries to minimize<br />
surface energy, so the slow<br />
growing faces will tend to<br />
dominate in the end<br />
E. Goeke, Fall 2006<br />
http://www.mtholyoke.edu/acad/geo/faculty/sd/zoned.html E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
http://www.engr.ku.edu/~rhale/ae510/lecture2/sld027.htm<br />
http://www.jwave.vt.edu/crcd/farkas/lectures/dislocations/tsld007.htm<br />
E. Goeke, Fall 2006<br />
Structural Defects<br />
• Point defects = xtal imperfections dealing with single<br />
locations in the structure<br />
– Vacant sites = Schottky defects<br />
– Atoms in the improper position = Frenkel defects<br />
– Extra atoms/ions = Interstitial defects<br />
– Atoms/ions substituted into the structure =<br />
Substitutional defects<br />
• Line defects = due to deformation at high temperatures;<br />
xtals deform along specific crystallographic planes & in<br />
preferred directions (slip system)<br />
– Edge dislocation = at right angles to the main stress<br />
– Screw dislocation = movement is parallel to the main<br />
stress<br />
• Planar defects = mismatch of the xtal structure along a<br />
surface<br />
– Grain boundaries<br />
– Stacking faults = layers are either out of position or<br />
sequence (e.g. ABABAABABA)<br />
– Antiphase boundaries = separate two+ sections of a xtal<br />
that are related by a simple translation<br />
• Both have the same crystallographic orientation<br />
http://wwwuser.gwdg.de/~upmp/neu/Arbeitsgruppen/Jooss/vizinal.html<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 5
Twinning<br />
• Twinning = symmetrical intergrowth of 2+ crystals of the<br />
same mineral<br />
– Can occur during growth, due to the application of<br />
stress to a pre-existing xtal, or because of a change in<br />
P/T conditions<br />
– Always adds symmetry, so it doesn’t occur along<br />
existing symmetry of the xtal<br />
• Operations that can cause twins:<br />
– Twin plane = reflection across a mirror plane<br />
– Twin axis = rotation around a line; always ⊥ to a lattice<br />
plane<br />
– Twin center = inversion through a point<br />
• Composition surface = plane/line along which lattice<br />
points are shared by the twins<br />
E. Goeke, Fall 2006<br />
Origin of Twinning<br />
• Three ways to get twins:<br />
1. Growh twins<br />
– Due to accidents during xtal growth that cause the a<br />
new xtal to be added to the face of a pre-existing xtal<br />
– 2 xtals share lattice points, but have different<br />
crystallographic orientations<br />
– Either contact or penetration twins<br />
2. Transformation twins<br />
– Pre-existing xtal changes form due to a change in<br />
pressure and/or temperature<br />
– Common in minerals that have different xtal structures<br />
& symmetry at different P’s & T’s (e.g. K-feldspars,<br />
pyroxenes)<br />
– As the xtal transforms into its new structure, different<br />
portions of the xtal arrange themselves in different<br />
• Contact twins = planar composition surface<br />
separates the 2+ xtals<br />
– Normally defined by a twin plane<br />
– No intergrowth of xtals<br />
– In 3+ xtals, if the composition surfaces are<br />
parallel to each other = polysnthetic twins<br />
(e.g. plagioclase)<br />
– If 3+ xtals have non-parallel composition<br />
surfaces = cyclical twins<br />
• Penetration twins = irregular composition<br />
surface<br />
– Due to a twin center or twin axis<br />
– Two xtals appear intergrown<br />
http://www.tulane.edu/~sanelson/eens211/twinning.htm<br />
E. Goeke, Fall 2006<br />
orientations E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
Common Twin Laws<br />
• Twin law = describes the twin operation<br />
& the orientation of the plane/axis along<br />
which the symmetry operation occurs<br />
– {hkl} = along a plane<br />
– [hkl] = axis of rotation<br />
• Triclinic<br />
– Mainly feldspars<br />
– Albite law = {010} polysynthetic<br />
twins ⊥ to b axis; diagnostic property<br />
of plagioclase<br />
– Pericline law = [010]; normally occurs<br />
during the transformation of<br />
orthoclase/sanidine to microcline & is<br />
present with albite twinning to form<br />
tartan twinning<br />
http://www.geolab.unc.edu/Petunia/<br />
IgMetAtlas/minerals/plagtwins.X.html<br />
E. Goeke, Fall 2006<br />
– Tartan twinning in<br />
microcline is due to the<br />
combination of albite &<br />
pericline twinning that occur<br />
when sanidine (high T)<br />
transforms to microcline<br />
(low T)<br />
3. Deformation twins<br />
– Atoms are pushed out of<br />
alignment due to stress<br />
– Commonly forms<br />
polysynthetic twins (e.g.<br />
plagioclase, calcile)<br />
• Monoclinic<br />
– Orthoclase & sanidine mainly<br />
– Manebach law = {001} in<br />
orthoclase; diagnostic when it<br />
occurs<br />
– Carlsbad law = [001], penetration<br />
twin in orthoclase; two xtals 180°<br />
from each other; most common type<br />
of twinning in orthoclase, so very<br />
diagnostic<br />
– Braveno law = {021}, contact twin<br />
in orthoclase<br />
– Swallow tail twins = {100},<br />
commonly found in gypsum<br />
http://www.geolab.unc.edu/Petunia/IgMetAtlas/minerals/microcline.X.html<br />
http://www.ucl.ac.uk/~ucfbrxs/PLM/calcite.html<br />
http://www.tulane.edu/~sanelson/eens211/twinning.htm<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 6
• Orthorhombic<br />
– Commonly twin on planes // to<br />
a prsim face<br />
– {110} cyclical twins =<br />
aragonite, chrysoberyl,<br />
cerrusite all have these twins;<br />
cyclical twins can cause the<br />
mineral to appear hexagonal<br />
– Staurolite law = staurolite is<br />
really monoclinic, but the β ~<br />
90°, so it appears orthorhombic<br />
• {031} forms right-angled<br />
cross<br />
• {231} form cross at ~60°<br />
• Isometric<br />
– Spinel law = {⎺⎺1⎺11}<br />
parallel to octahedron;<br />
common in spinel<br />
– [111] = twin axis ⊥ to octahedral face that<br />
adds a 3-fold rotational symmetry<br />
– Iron cross = [001], interpenetration of two<br />
pyritohedrons; occurs in pyrite; twinning<br />
causes an apparent 4-fold symmetry<br />
http://www.tulane.edu/~sanelson/eens211/twinning.htm<br />
http://www.ged.rwth-aachen.de/Ww/projects/rexx/Urai+86Recrystallization/Urai+86Recrystallization5.htm<br />
E. Goeke, Fall 2006<br />
http://www.tulane.edu/~sanelson/eens211/twinning.htm<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006<br />
• Tetragonal<br />
– {011} cyclical contact twins =<br />
common in rutile & cassiterite<br />
• Hexagonal<br />
– Calcilte twins = two types of<br />
contact twins<br />
• {0001}<br />
• {01⎺12} can also occur as<br />
polysynthetic twins due to<br />
deformation<br />
– In quartz:<br />
• Brazil law = {11⎺20} penetration<br />
twins due to transformation<br />
• Dauphiné law = [0001]<br />
penetration twin due to<br />
transformation<br />
• Japanese law = {11⎺22} contact<br />
twin during growth http://www.tulane.edu/~sanelson/eens211/twinning.htm<br />
Postcrystallization Processes<br />
E. Goeke, Fall 2006<br />
• Once a mineral has xtalized, it may become unstable due to<br />
a change in P and/or T, the addition of a fluid phase, or a<br />
change in bulk composition<br />
• Several processes may occur to change the metastable<br />
minerals into more stable phases:<br />
– Ordering -> may cause changes in the unit cell<br />
dimensions and/or the symmetry (e.g. pyroxenes,<br />
amphiboles, K-feldspars)<br />
– Twinning -> transformation twinning (e.g. K-feldspar,<br />
leucite)<br />
– Recrystallization -> to reduce the surface energy by<br />
removing structure defects, mineral grains change<br />
shape and/or size<br />
– Exsolution -> occurs in minerals that have complete<br />
solid solution at high T, but only partial solid solution<br />
at low T’s<br />
http://tesla.jcu.edu.au/Schools/Earth/EA1001/<strong>Mineral</strong>ogy/<strong>Mineral</strong>s/Feldspars.html<br />
E. Goeke, Fall 2006<br />
At high T’s, we will have 1 feldspar,<br />
but at lower T’s 2 feldspars will be<br />
present (proportion with Lever rule)<br />
http://www.tulane.edu/~sanelson/eens211/2compphasdiag.html<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 7
K-feldspar w/<br />
blebs of<br />
plagioclase<br />
http://www.geolab.unc.edu/Petunia/IgMetAtlas/plutonic-micro%7F/perthite1.X.html http://www.geolab.unc.edu/Petunia/IgMetAtlas/plutonic-micro%7F/perthite2.X.html<br />
Opx w/<br />
blebs of cpx<br />
http://www.ucl.ac.uk/~ucfbrxs/PLM/opx.html<br />
Radioactivity & <strong>Mineral</strong>s<br />
• Radioactive decay can also cause post-crystallization<br />
alteration<br />
• 40 K and 14 C are two common radioactive constituents in<br />
minerals<br />
• U & Th may also be found in various minerals, but<br />
usually not as a major component<br />
• The decay of one element to another can cause space<br />
issues (e.g. 40 K -> 40 Ar or 40 Ca, where neither daughter<br />
element is the same size or charge as the parent)<br />
• Decay can also cause structural damage to the mineral<br />
grain as the released alpha particle disrupts the normal<br />
structure (cause of metamict minerals)<br />
• Released alpha particles may also leave the original<br />
grain that contained the parent mineral & enter a<br />
neighboring grain, which can cause a pleochroic halo to<br />
form around the primary mineral<br />
E. Goeke, Fall 2006<br />
http://www.ucl.ac.uk/~ucfbrxs/PLM/zircon.html<br />
E. Goeke, Fall 2006<br />
Pseudomorphism<br />
• Pseudomorphism = replacement of<br />
a mineral grain by another mineral,<br />
but the 2nd mineral retains the<br />
characteristic grain outline of the 1st<br />
mineral<br />
– Three types:<br />
1. Substitution = direct<br />
replacement of one mineral by a<br />
second (e.g. chlorite after<br />
garnet)<br />
2. Encrustation = thin crust of a<br />
new mineral forms on the<br />
surface of a preexisting mineral,<br />
which is followed by the<br />
removal of the 1st grain<br />
3. Alteration = partial removal of<br />
original mineral & only partial<br />
replacement by the new mineral http://www.ucl.ac.uk/~ucfbrxs/PLM/opx.html<br />
E. Goeke, Fall 2006<br />
E. Goeke, Fall 2006, 12:041 <strong>Mineral</strong>ogy 8