Baldwin's Rules - Department of Medicinal Chemistry
Baldwin's Rules - Department of Medicinal Chemistry
Baldwin's Rules - Department of Medicinal Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chemical Reviews REVIEW<br />
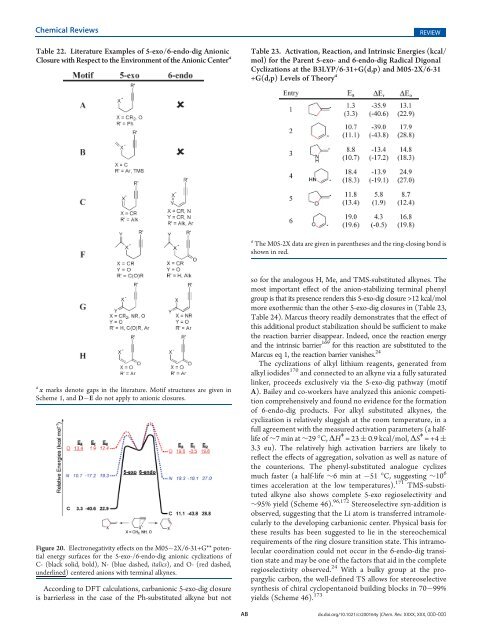
Table 22. Literature Examples <strong>of</strong> 5-exo/6-endo-dig Anionic<br />
Closure with Respect to the Environment <strong>of</strong> the Anionic Center a<br />
a x marks denote gaps in the literature. Motif structures are given in<br />
Scheme 1, and D E do not apply to anionic closures.<br />
Figure 20. Electronegativity effects on the M05 2X/6-31+G** potential<br />
energy surfaces for the 5-exo-/6-endo-dig anionic cyclizations <strong>of</strong><br />
C- (black solid, bold), N- (blue dashed, italics), and O- (red dashed,<br />
underlined) centered anions with terminal alkynes.<br />
According to DFT calculations, carbanionic 5-exo-dig closure<br />
is barrierless in the case <strong>of</strong> the Ph-substituted alkyne but not<br />
Table 23. Activation, Reaction, and Intrinsic Energies (kcal/<br />
mol) for the Parent 5-exo- and 6-endo-dig Radical Digonal<br />
Cyclizations at the B3LYP/6-31+G(d,p) and M05-2X/6-31<br />
+G(d,p) Levels <strong>of</strong> Theory a<br />
a<br />
The M05-2X data are given in parentheses and the ring-closing bond is<br />
shown in red.<br />
so for the analogous H, Me, and TMS-substituted alkynes. The<br />
most important effect <strong>of</strong> the anion-stabilizing terminal phenyl<br />
group is that its presence renders this 5-exo-dig closure >12 kcal/mol<br />
more exothermic than the other 5-exo-dig closures in (Table 23,<br />
Table 24). Marcus theory readily demonstrates that the effect <strong>of</strong><br />
this additional product stabilization should be sufficient to make<br />
the reaction barrier disappear. Indeed, once the reaction energy<br />
and the intrinsic barrier 169 for this reaction are substituted to the<br />
Marcus eq 1, the reaction barrier vanishes. 24<br />
The cyclizations <strong>of</strong> alkyl lithium reagents, generated from<br />
alkyl iodides 170 and connected to an alkyne via a fully saturated<br />
linker, proceeds exclusively via the 5-exo-dig pathway (motif<br />
A). Bailey and co-workers have analyzed this anionic competition<br />
comprehensively and found no evidence for the formation<br />
<strong>of</strong> 6-endo-dig products. For alkyl substituted alkynes, the<br />
cyclization is relatively sluggish at the room temperature, in a<br />
full agreement with the measured activation parameters (a halflife<br />
<strong>of</strong> ∼7minat∼29 °C, ΔH q =23( 0.9 kcal/mol, ΔS q =+4(<br />
3.3 eu). The relatively high activation barriers are likely to<br />
reflect the effects <strong>of</strong> aggregation, solvation as well as nature <strong>of</strong><br />
the counterions. The phenyl-substituted analogue cyclizes<br />
much faster (a half-life ∼6 minat 51 °C, suggesting ∼10 6<br />
times acceleration at the low temperatures). 171 TMS-substituted<br />
alkyne also shows complete 5-exo regioselectivity and<br />
∼95% yield (Scheme 46). 96,172 Stereoselective syn-addition is<br />
observed, suggesting that the Li atom is transferred intramolecularly<br />
to the developing carbanionic center. Physical basis for<br />
these results has been suggested to lie in the stereochemical<br />
requirements <strong>of</strong> the ring closure transition state. This intramolecular<br />
coordination could not occur in the 6-endo-dig transition<br />
state and may be one <strong>of</strong> the factors that aid in the complete<br />
regioselectivity observed. 24 With a bulky group at the propargylic<br />
carbon, the well-defined TS allows for stereoselective<br />
synthesis <strong>of</strong> chiral cyclopentanoid building blocks in 70 99%<br />
yields (Scheme 46). 173<br />
AB dx.doi.org/10.1021/cr200164y |Chem. Rev. XXXX, XXX, 000–000