"Initiators, Free-Radical". In: Encyclopedia of Polymer Science and ...
"Initiators, Free-Radical". In: Encyclopedia of Polymer Science and ...
"Initiators, Free-Radical". In: Encyclopedia of Polymer Science and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Vol. 6 INITIATORS, FREE-RADICAL 577<br />
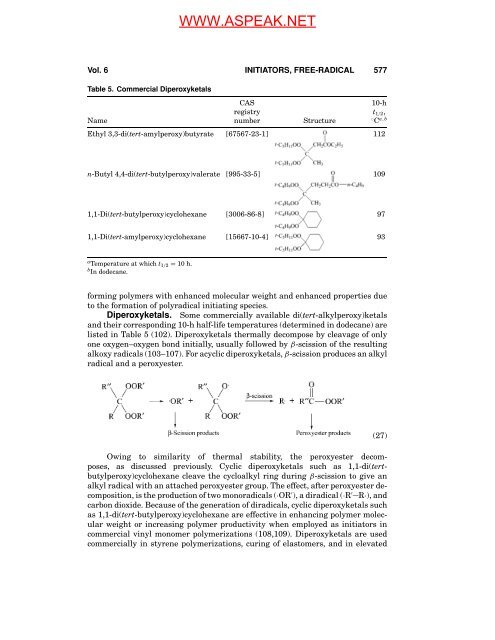
Table 5. Commercial Diperoxyketals<br />
CAS 10-h<br />
registry t1/2,<br />
Name number Structure ◦ C a,b<br />
Ethyl 3,3-di(tert-amylperoxy)butyrate [67567-23-1] 112<br />
n-Butyl 4,4-di(tert-butylperoxy)valerate [995-33-5] 109<br />
1,1-Di(tert-butylperoxy)cyclohexane [3006-86-8] 97<br />
1,1-Di(tert-amylperoxy)cyclohexane [15667-10-4] 93<br />
a Temperature at which t1/2 = 10 h.<br />
b <strong>In</strong> dodecane.<br />
WWW.ASPEAK.NET<br />
forming polymers with enhanced molecular weight <strong>and</strong> enhanced properties due<br />
to the formation <strong>of</strong> polyradical initiating species.<br />
Diperoxyketals. Some commercially available di(tert-alkylperoxy)ketals<br />
<strong>and</strong> their corresponding 10-h half-life temperatures (determined in dodecane) are<br />
listed in Table 5 (102). Diperoxyketals thermally decompose by cleavage <strong>of</strong> only<br />
one oxygen–oxygen bond initially, usually followed by β-scission <strong>of</strong> the resulting<br />
alkoxy radicals (103–107). For acyclic diperoxyketals, β-scission produces an alkyl<br />
radical <strong>and</strong> a peroxyester.<br />
Owing to similarity <strong>of</strong> thermal stability, the peroxyester decomposes,<br />
as discussed previously. Cyclic diperoxyketals such as 1,1-di(tertbutylperoxy)cyclohexane<br />
cleave the cycloalkyl ring during β-scission to give an<br />
alkyl radical with an attached peroxyester group. The effect, after peroxyester decomposition,<br />
is the production <strong>of</strong> two monoradicals (·OR ′ ), a diradical (·R ′ R·), <strong>and</strong><br />
carbon dioxide. Because <strong>of</strong> the generation <strong>of</strong> diradicals, cyclic diperoxyketals such<br />
as 1,1-di(tert-butylperoxy)cyclohexane are effective in enhancing polymer molecular<br />
weight or increasing polymer productivity when employed as initiators in<br />
commercial vinyl monomer polymerizations (108,109). Diperoxyketals are used<br />
commercially in styrene polymerizations, curing <strong>of</strong> elastomers, <strong>and</strong> in elevated<br />
(27)