"Initiators, Free-Radical". In: Encyclopedia of Polymer Science and ...
"Initiators, Free-Radical". In: Encyclopedia of Polymer Science and ...
"Initiators, Free-Radical". In: Encyclopedia of Polymer Science and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Vol. 6 INITIATORS, FREE-RADICAL 579<br />
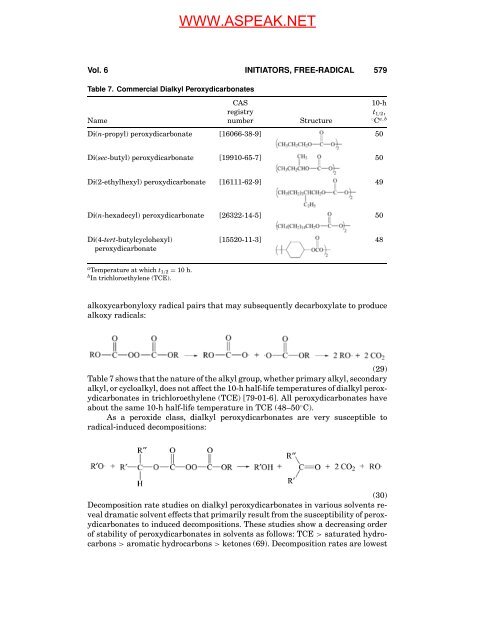
Table 7. Commercial Dialkyl Peroxydicarbonates<br />
CAS 10-h<br />
registry t1/2,<br />
Name number Structure ◦ C a,b<br />
Di(n-propyl) peroxydicarbonate [16066-38-9] 50<br />
Di(sec-butyl) peroxydicarbonate [19910-65-7] 50<br />
Di(2-ethylhexyl) peroxydicarbonate [16111-62-9] 49<br />
Di(n-hexadecyl) peroxydicarbonate [26322-14-5] 50<br />
Di(4-tert-butylcyclohexyl) [15520-11-3] 48<br />
peroxydicarbonate<br />
a Temperature at which t1/2 = 10 h.<br />
b <strong>In</strong> trichloroethylene (TCE).<br />
WWW.ASPEAK.NET<br />
alkoxycarbonyloxy radical pairs that may subsequently decarboxylate to produce<br />
alkoxy radicals:<br />
(29)<br />
Table 7 shows that the nature <strong>of</strong> the alkyl group, whether primary alkyl, secondary<br />
alkyl, or cycloalkyl, does not affect the 10-h half-life temperatures <strong>of</strong> dialkyl peroxydicarbonates<br />
in trichloroethylene (TCE) [79-01-6]. All peroxydicarbonates have<br />
about the same 10-h half-life temperature in TCE (48–50 ◦ C).<br />
As a peroxide class, dialkyl peroxydicarbonates are very susceptible to<br />
radical-induced decompositions:<br />
(30)<br />
Decomposition rate studies on dialkyl peroxydicarbonates in various solvents reveal<br />
dramatic solvent effects that primarily result from the susceptibility <strong>of</strong> peroxydicarbonates<br />
to induced decompositions. These studies show a decreasing order<br />
<strong>of</strong> stability <strong>of</strong> peroxydicarbonates in solvents as follows: TCE > saturated hydrocarbons<br />
> aromatic hydrocarbons > ketones (69). Decomposition rates are lowest