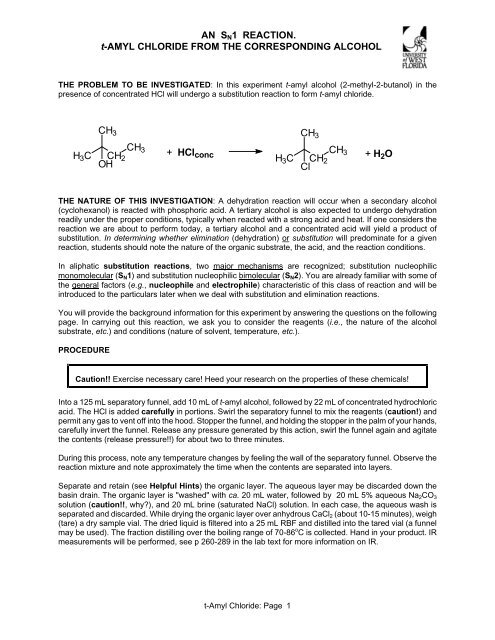
AN SN1 REACTION. t-AMYL CHLORIDE FROM THE ...
AN SN1 REACTION. t-AMYL CHLORIDE FROM THE ...
AN SN1 REACTION. t-AMYL CHLORIDE FROM THE ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>AN</strong> <strong>SN1</strong> <strong>REACTION</strong>.<br />
t-<strong>AMYL</strong> <strong>CHLORIDE</strong> <strong>FROM</strong> <strong>THE</strong> CORRESPONDING ALCOHOL<br />
<strong>THE</strong> PROBLEM TO BE INVESTIGATED: In this experiment t-amyl alcohol (2-methyl-2-butanol) in the<br />
presence of concentrated HCl will undergo a substitution reaction to form t-amyl chloride.<br />
CH3<br />
CH3<br />
H3C CH2<br />
OH<br />
<strong>THE</strong> NATURE OF THIS INVESTIGATION: A dehydration reaction will occur when a secondary alcohol<br />
(cyclohexanol) is reacted with phosphoric acid. A tertiary alcohol is also expected to undergo dehydration<br />
readily under the proper conditions, typically when reacted with a strong acid and heat. If one considers the<br />
reaction we are about to perform today, a tertiary alcohol and a concentrated acid will yield a product of<br />
substitution. In determining whether elimination (dehydration) or substitution will predominate for a given<br />
reaction, students should note the nature of the organic substrate, the acid, and the reaction conditions.<br />
In aliphatic substitution reactions, two major mechanisms are recognized; substitution nucleophilic<br />
monomolecular (<strong>SN1</strong>) and substitution nucleophilic bimolecular (SN2). You are already familiar with some of<br />
the general factors (e.g., nucleophile and electrophile) characteristic of this class of reaction and will be<br />
introduced to the particulars later when we deal with substitution and elimination reactions.<br />
You will provide the background information for this experiment by answering the questions on the following<br />
page. In carrying out this reaction, we ask you to consider the reagents (i.e., the nature of the alcohol<br />
substrate, etc.) and conditions (nature of solvent, temperature, etc.).<br />
PROCEDURE<br />
+ HClconc<br />
Caution!! Exercise necessary care! Heed your research on the properties of these chemicals!<br />
Into a 125 mL separatory funnel, add 10 mL of t-amyl alcohol, followed by 22 mL of concentrated hydrochloric<br />
acid. The HCl is added carefully in portions. Swirl the separatory funnel to mix the reagents (caution!) and<br />
permit any gas to vent off into the hood. Stopper the funnel, and holding the stopper in the palm of your hands,<br />
carefully invert the funnel. Release any pressure generated by this action, swirl the funnel again and agitate<br />
the contents (release pressure!!) for about two to three minutes.<br />
During this process, note any temperature changes by feeling the wall of the separatory funnel. Observe the<br />
reaction mixture and note approximately the time when the contents are separated into layers.<br />
Separate and retain (see Helpful Hints) the organic layer. The aqueous layer may be discarded down the<br />
basin drain. The organic layer is "washed" with ca. 20 mL water, followed by 20 mL 5% aqueous Na2CO3<br />
solution (caution!!, why?), and 20 mL brine (saturated NaCl) solution. In each case, the aqueous wash is<br />
separated and discarded. While drying the organic layer over anhydrous CaCl2 (about 10-15 minutes), weigh<br />
(tare) a dry sample vial. The dried liquid is filtered into a 25 mL RBF and distilled into the tared vial (a funnel<br />
may be used). The fraction distilling over the boiling range of 70-86 o C is collected. Hand in your product. IR<br />
measurements will be performed, see p 260-289 in the lab text for more information on IR.<br />
H3C<br />
t-Amyl Chloride: Page 1<br />
CH3<br />
CH2<br />
Cl<br />
CH 3<br />
+ H2O
Helpful Hints - t-Amyl chloride<br />
* Whenever a separatory funnel is used, do not discard any fraction unless you can account for your<br />
product. An easy method to ascertain which layer is which is the following: Add about 2-3 mL of water<br />
into a test tube, and place the test tube lip around the "takeout arm" of your separatory funnel. The<br />
bottom layer is released through the stopcock into the test tube containing the water. Upon agitation,<br />
observe whether the mixture is homogeneous. By deductive reasoning, you now know (hopefully)<br />
which layer is which!!<br />
SYN<strong>THE</strong>SIS OF tert-<strong>AMYL</strong> <strong>CHLORIDE</strong> - HOMEWORK QUESTIONS<br />
Tertiary alkyl bromides can be prepared from the corresponding alcohol by the reaction with concentrated<br />
hydrobromic acid instead of conc.. hydrochloric acid as shown by the following equation.<br />
(1) Consider the above reaction using 25.0 mL of tert-butyl alcohol (d = 0.786 g/mL) with 60.0 mL of<br />
concentrated hydrobromic acid (d = 1.49 g/mL, 47.0% HBr). On a separate sheet calculate the theoretical<br />
yield in grams and the percent yield for a reaction that produced 26.1 g of tert-butyl bromide. Clearly show<br />
the set ups to determine the limiting reactant and other calculations using proper units and significant<br />
figures.<br />
Consult your textbook for the following TWO synthesis. Keep in mind that conc. HCl and conc. HBr<br />
will NOT give good yields of alkyl halides by the reaction with primary and secondary alcohols.<br />
(2) Give the balanced equation to prepare chlorocyclohexane in good yield from cyclohexanol.<br />
(3) Give the balanced equation for the preparation in good yield of 1-bromopentane from 1-pentanol.<br />
(4) The reverse of the reaction you performed in the lab can also occur. Under the proper conditions,<br />
tertiary alkyl halides may undergo a hydrolysis reaction to form an alcohol and a hydrogen halide.<br />
Complete and balance the following equation. (Remember the General II experiment?)<br />
t-Amyl Chloride: Page 2