- Page 2 and 3: Chemistry for Pharmacy Students Gen
- Page 4: This book is dedicated to pharmacy
- Page 7 and 8: viii CONTENTS 5.5 Substitution reac
- Page 9 and 10: x PREFACE countries. Therefore, the
- Page 12 and 13: 1 Introduction Learning objectives
- Page 14 and 15: Whatever the source is, chemistry i
- Page 16 and 17: 1.2 PHYSICAL PROPERTIES OF DRUG MOL
- Page 18 and 19: Each acid has a conjugate base, and
- Page 20 and 21: 1.2 PHYSICAL PROPERTIES OF DRUG MOL
- Page 22 and 23: CH3CH3 ðEthaneÞ !CH3NH2 ðMethyla
- Page 24 and 25: takes place. K a ¼ K eq½H2OŠ ¼
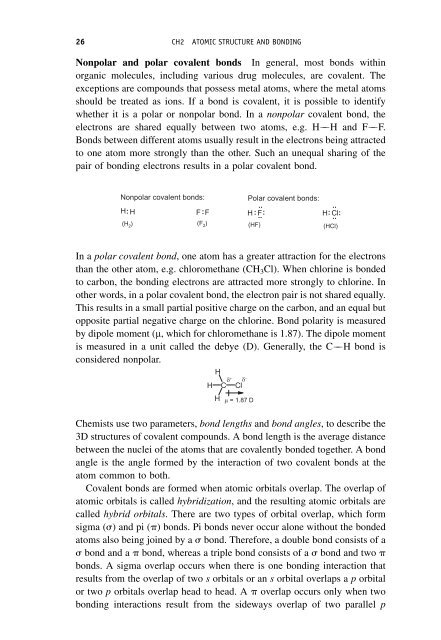
- Page 26: Recommended further reading RECOMME
- Page 29 and 30: 18 CH2 ATOMIC STRUCTURE AND BONDING
- Page 31 and 32: 20 CH2 ATOMIC STRUCTURE AND BONDING
- Page 33 and 34: 22 CH2 ATOMIC STRUCTURE AND BONDING
- Page 35: 24 CH2 ATOMIC STRUCTURE AND BONDING
- Page 39 and 40: 28 CH2 ATOMIC STRUCTURE AND BONDING
- Page 41 and 42: 30 CH2 ATOMIC STRUCTURE AND BONDING
- Page 43 and 44: 32 CH2 ATOMIC STRUCTURE AND BONDING
- Page 46 and 47: 3 Stereochemistry Learning objectiv
- Page 48 and 49: dimensional orientation of the hydr
- Page 50 and 51: H H H H H H H H H H H C 3 Butane Am
- Page 52 and 53: Again, in reality, cyclohexane is n
- Page 54 and 55: not chiral. With achiral molecules,
- Page 56 and 57: 3.2 ISOMERISM 45 When we have a pai
- Page 58 and 59: ( ). This D and L system is common
- Page 60 and 61: HO H 4 1 2COOH CH2OH 3 (R)-2,3-Dihy
- Page 62 and 63: Geometrical isomers H H H H Cl Cl C
- Page 64 and 65: 3.3 SIGNIFICANCE OF STEREOISOMERISM
- Page 66 and 67: H H * * (+)-Limonene (in orange) (
- Page 68 and 69: 3.7 CHIRAL COMPOUNDS THAT DO NOT HA
- Page 70 and 71: 4 Organic functional groups Learnin
- Page 72 and 73: ðContinuedÞ Name General structur
- Page 74 and 75: 4.3 ALKANES, CYCLOALKANES AND THEIR
- Page 76 and 77: drawn as solid wedges, and those po
- Page 78 and 79: 4.3 ALKANES, CYCLOALKANES AND THEIR
- Page 80 and 81: CH4 + 2 O2 CO2 + 2 H2O CH3CH2CH + 2
- Page 82 and 83: Reactivity of alkyl halides RHC CH2
- Page 84 and 85: Alkanes can be prepared from alkyl
- Page 86 and 87:
4.3 ALKANES, CYCLOALKANES AND THEIR
- Page 88 and 89:
H OH OH H i. BH3 .THF H2O, H2SO4 or
- Page 90 and 91:
Nomenclature of thiols The nomencla
- Page 92 and 93:
H2C CH2 CH3CH CH2 CH3CH2CH CH2 O O
- Page 94 and 95:
Nomenclature of amines Aliphatic am
- Page 96 and 97:
aldehydes and ketones, followed by
- Page 98 and 99:
O C2H5 C 4.3 ALKANES, CYCLOALKANES
- Page 100 and 101:
earlier, alcohols can be prepared f
- Page 102 and 103:
Aliphatic dicarboxylic acids are na
- Page 104 and 105:
aldehydes (see Section 5.7.11), ozo
- Page 106 and 107:
4.3.14 Acid chlorides The functiona
- Page 108 and 109:
Preparation of acid anhydrides Anhy
- Page 110 and 111:
Reactions of esters Esters are less
- Page 112 and 113:
espectively (see Section 5.5.5). Th
- Page 114 and 115:
eagent or organolithium produces ke
- Page 116 and 117:
unsaturated groups are called the v
- Page 118 and 119:
Alkenes are obtained from selective
- Page 120 and 121:
equires two hybrid orbitals to bond
- Page 122 and 123:
addition reactions, e.g. hydrogenat
- Page 124 and 125:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 126 and 127:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 128 and 129:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 130 and 131:
form two doughnut-shaped clouds of
- Page 132 and 133:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 134 and 135:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 136 and 137:
the overlap of a p orbital on the s
- Page 138 and 139:
electrons from the ring through the
- Page 140 and 141:
CH 2 Br Bromomethylbenzene 4.6.10 P
- Page 142 and 143:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 144 and 145:
A number of other reactions can als
- Page 146 and 147:
Physical properties of aniline 4.6
- Page 148 and 149:
From chlorobenzene Treatment of chl
- Page 150 and 151:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 152 and 153:
4.6 AROMATIC COMPOUNDS AND THEIR DE
- Page 154 and 155:
4.7 HETEROCYCLIC COMPOUNDS AND THEI
- Page 156 and 157:
4.7 HETEROCYCLIC COMPOUNDS AND THEI
- Page 158 and 159:
4.7.4 PYRROLE, FURAN AND THIOPHENE:
- Page 160 and 161:
Furan is synthesized by decarbonyla
- Page 162 and 163:
N H S + HNO 3 + HNO 3 Acetic anhydr
- Page 164 and 165:
dipole moment of pyridine is 1.57 D
- Page 166 and 167:
N Cl 2-Chloropyridine Br N OMe 4-Br
- Page 168 and 169:
R' R NH 2 O + R'' O X Base R' R O H
- Page 170 and 171:
for isoxazole, pyrazole and isothia
- Page 172 and 173:
A number of drug molecules contain
- Page 174 and 175:
4.7.9 Purine Purine contains a pyri
- Page 176 and 177:
4.7.10 Quinoline and isoquinoline 4
- Page 178 and 179:
MeO MeO MeO MeO NH 2 β-Phenylethyl
- Page 180 and 181:
at room temperature, and posseses a
- Page 182 and 183:
In the nucleotides, while the heter
- Page 184 and 185:
4.8.2 Structure of nucleic acids Pr
- Page 186 and 187:
Sugar _ phosphate backbone O O P O
- Page 188 and 189:
Transcription: synthesis of RNA 4.8
- Page 190 and 191:
sample is obtained from a crime sce
- Page 192 and 193:
AVK (one letter). The ends of a pep
- Page 194 and 195:
Carbon CO 2 + H 2 O 4.9 AMINO ACIDS
- Page 196 and 197:
4.10 IMPORTANCE OF FUNCTIONAL GROUP
- Page 198 and 199:
4.10 IMPORTANCE OF FUNCTIONAL GROUP
- Page 200:
Certain functional groups in drug m
- Page 203 and 204:
192 CH5 ORGANIC REACTIONS Reaction
- Page 205 and 206:
194 CH5 ORGANIC REACTIONS H C 3 H +
- Page 207 and 208:
196 CH5 ORGANIC REACTIONS brominati
- Page 209 and 210:
198 CH5 ORGANIC REACTIONS General r
- Page 211 and 212:
200 CH5 ORGANIC REACTIONS Electroph
- Page 213 and 214:
202 CH5 ORGANIC REACTIONS addition
- Page 215 and 216:
204 CH5 ORGANIC REACTIONS Br. + Br.
- Page 217 and 218:
206 CH5 ORGANIC REACTIONS CH3CH CH2
- Page 219 and 220:
208 CH5 ORGANIC REACTIONS Hydrobora
- Page 221 and 222:
210 CH5 ORGANIC REACTIONS p bond, a
- Page 223 and 224:
212 CH5 ORGANIC REACTIONS Mechanism
- Page 225 and 226:
214 CH5 ORGANIC REACTIONS Addition
- Page 227 and 228:
216 CH5 ORGANIC REACTIONS molecule
- Page 229 and 230:
218 CH5 ORGANIC REACTIONS O R C Y Y
- Page 231 and 232:
220 CH5 ORGANIC REACTIONS condition
- Page 233 and 234:
222 CH5 ORGANIC REACTIONS Aldol con
- Page 235 and 236:
224 CH5 ORGANIC REACTIONS remove th
- Page 237 and 238:
226 CH5 ORGANIC REACTIONS formation
- Page 239 and 240:
228 CH5 ORGANIC REACTIONS Mechanism
- Page 241 and 242:
230 CH5 ORGANIC REACTIONS Stereoche
- Page 243 and 244:
232 CH5 ORGANIC REACTIONS E1 versus
- Page 245 and 246:
234 CH5 ORGANIC REACTIONS solvent i
- Page 247 and 248:
236 CH5 ORGANIC REACTIONS configura
- Page 249 and 250:
238 CH5 ORGANIC REACTIONS the base,
- Page 251 and 252:
240 CH5 ORGANIC REACTIONS Mechanism
- Page 253 and 254:
242 CH5 ORGANIC REACTIONS The react
- Page 255 and 256:
244 CH5 ORGANIC REACTIONS Conversio
- Page 257 and 258:
246 CH5 ORGANIC REACTIONS Mechanism
- Page 259 and 260:
248 CH5 ORGANIC REACTIONS Mechanism
- Page 261 and 262:
250 CH5 ORGANIC REACTIONS Conversio
- Page 263 and 264:
252 CH5 ORGANIC REACTIONS Preparati
- Page 265 and 266:
254 CH5 ORGANIC REACTIONS Nucleophi
- Page 267 and 268:
256 CH5 ORGANIC REACTIONS In the ca
- Page 269 and 270:
258 CH5 ORGANIC REACTIONS Cl 2 , Fe
- Page 271 and 272:
260 CH5 ORGANIC REACTIONS Step 3. L
- Page 273 and 274:
262 CH5 ORGANIC REACTIONS : O: R C
- Page 275 and 276:
264 CH5 ORGANIC REACTIONS water. Th
- Page 277 and 278:
266 CH5 ORGANIC REACTIONS Preparati
- Page 279 and 280:
268 CH5 ORGANIC REACTIONS work-up.
- Page 281 and 282:
270 CH5 ORGANIC REACTIONS (C5H5NH +
- Page 283 and 284:
272 CH5 ORGANIC REACTIONS OH Cyclop
- Page 285 and 286:
274 CH5 ORGANIC REACTIONS hydride s
- Page 287 and 288:
276 CH5 ORGANIC REACTIONS 5.7.20 Re
- Page 289 and 290:
278 CH5 ORGANIC REACTIONS Preparati
- Page 291 and 292:
280 CH5 ORGANIC REACTIONS Cyclic co
- Page 293 and 294:
282 CH5 ORGANIC REACTIONS with a mi
- Page 295 and 296:
284 CH6 NATURAL PRODUCT CHEMISTRY 6
- Page 297 and 298:
286 CH6 NATURAL PRODUCT CHEMISTRY t
- Page 299 and 300:
288 CH6 NATURAL PRODUCT CHEMISTRY L
- Page 301 and 302:
290 CH6 NATURAL PRODUCT CHEMISTRY C
- Page 303 and 304:
292 CH6 NATURAL PRODUCT CHEMISTRY O
- Page 305 and 306:
294 CH6 NATURAL PRODUCT CHEMISTRY p
- Page 307 and 308:
296 CH6 NATURAL PRODUCT CHEMISTRY I
- Page 309 and 310:
298 CH6 NATURAL PRODUCT CHEMISTRY T
- Page 311 and 312:
300 CH6 NATURAL PRODUCT CHEMISTRY P
- Page 313 and 314:
302 CH6 NATURAL PRODUCT CHEMISTRY M
- Page 315 and 316:
304 CH6 NATURAL PRODUCT CHEMISTRY a
- Page 317 and 318:
306 CH6 NATURAL PRODUCT CHEMISTRY h
- Page 319 and 320:
308 CH6 NATURAL PRODUCT CHEMISTRY H
- Page 321 and 322:
310 CH6 NATURAL PRODUCT CHEMISTRY H
- Page 323 and 324:
312 CH6 NATURAL PRODUCT CHEMISTRY C
- Page 325 and 326:
314 CH6 NATURAL PRODUCT CHEMISTRY 6
- Page 327 and 328:
316 CH6 NATURAL PRODUCT CHEMISTRY g
- Page 329 and 330:
318 CH6 NATURAL PRODUCT CHEMISTRY M
- Page 331 and 332:
320 CH6 NATURAL PRODUCT CHEMISTRY a
- Page 333 and 334:
322 CH6 NATURAL PRODUCT CHEMISTRY T
- Page 335 and 336:
324 CH6 NATURAL PRODUCT CHEMISTRY k
- Page 337 and 338:
326 CH6 NATURAL PRODUCT CHEMISTRY S
- Page 339 and 340:
328 CH6 NATURAL PRODUCT CHEMISTRY B
- Page 341 and 342:
330 CH6 NATURAL PRODUCT CHEMISTRY h
- Page 343 and 344:
332 CH6 NATURAL PRODUCT CHEMISTRY (
- Page 345 and 346:
334 CH6 NATURAL PRODUCT CHEMISTRY G
- Page 347 and 348:
336 CH6 NATURAL PRODUCT CHEMISTRY (
- Page 349 and 350:
338 CH6 NATURAL PRODUCT CHEMISTRY (
- Page 351 and 352:
340 CH6 NATURAL PRODUCT CHEMISTRY (
- Page 353 and 354:
342 CH6 NATURAL PRODUCT CHEMISTRY (
- Page 355 and 356:
344 CH6 NATURAL PRODUCT CHEMISTRY S
- Page 357 and 358:
346 CH6 NATURAL PRODUCT CHEMISTRY P
- Page 359 and 360:
348 CH6 NATURAL PRODUCT CHEMISTRY M
- Page 361 and 362:
350 CH6 NATURAL PRODUCT CHEMISTRY B
- Page 363 and 364:
352 CH6 NATURAL PRODUCT CHEMISTRY i
- Page 365 and 366:
354 CH6 NATURAL PRODUCT CHEMISTRY I
- Page 367 and 368:
356 CH6 NATURAL PRODUCT CHEMISTRY H
- Page 369 and 370:
358 CH6 NATURAL PRODUCT CHEMISTRY C
- Page 371 and 372:
360 CH6 NATURAL PRODUCT CHEMISTRY s
- Page 373 and 374:
362 CH6 NATURAL PRODUCT CHEMISTRY N
- Page 375 and 376:
364 CH6 NATURAL PRODUCT CHEMISTRY c
- Page 377 and 378:
366 CH6 NATURAL PRODUCT CHEMISTRY a
- Page 379 and 380:
368 CH6 NATURAL PRODUCT CHEMISTRY F
- Page 381 and 382:
370 CH6 NATURAL PRODUCT CHEMISTRY R
- Page 383 and 384:
372 INDEX Amino sugar 304, 305, 317
- Page 385 and 386:
374 INDEX Closed shell configuratio
- Page 387 and 388:
376 INDEX Friedel-Crafts alkylation
- Page 389 and 390:
378 INDEX Lincomycin 318 Linum usit
- Page 391 and 392:
380 INDEX Polymerization 106, 110 P
- Page 393 and 394:
382 INDEX Sulphanilamide 140 Sulpha