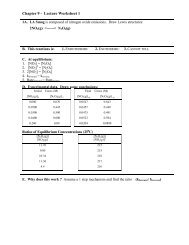
Experiment 4 Studying the Spectrochemical Series: Crystal Fields of ...
Experiment 4 Studying the Spectrochemical Series: Crystal Fields of ...
Experiment 4 Studying the Spectrochemical Series: Crystal Fields of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
O<br />
H 2N NH 2<br />
O<br />
H3C C CH3 H2 + H 2O 2 NH 3 + CO 2<br />
O<br />
H<br />
O<br />
O<br />
H3C C CH3 H<br />
O<br />
O<br />
H3C C CH3 H<br />
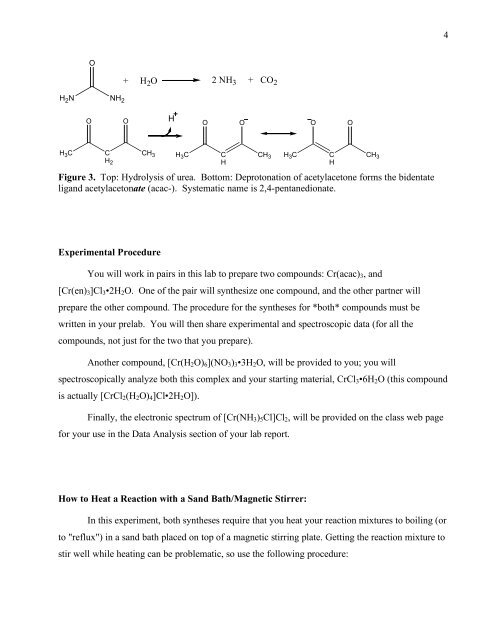
Figure 3. Top: Hydrolysis <strong>of</strong> urea. Bottom: Deprotonation <strong>of</strong> acetylacetone forms <strong>the</strong> bidentate<br />
ligand acetylacetonate (acac-). Systematic name is 2,4-pentanedionate.<br />
<strong>Experiment</strong>al Procedure<br />
You will work in pairs in this lab to prepare two compounds: Cr(acac)3, and<br />
[Cr(en)3]Cl3•2H2O. One <strong>of</strong> <strong>the</strong> pair will syn<strong>the</strong>size one compound, and <strong>the</strong> o<strong>the</strong>r partner will<br />
prepare <strong>the</strong> o<strong>the</strong>r compound. The procedure for <strong>the</strong> syn<strong>the</strong>ses for *both* compounds must be<br />
written in your prelab. You will <strong>the</strong>n share experimental and spectroscopic data (for all <strong>the</strong><br />
compounds, not just for <strong>the</strong> two that you prepare).<br />
Ano<strong>the</strong>r compound, [Cr(H2O)6](NO3)3•3H2O, will be provided to you; you will<br />
spectroscopically analyze both this complex and your starting material, CrCl3•6H2O (this compound<br />
is actually [CrCl2(H2O)4]Cl•2H2O]).<br />
Finally, <strong>the</strong> electronic spectrum <strong>of</strong> [Cr(NH3)5Cl]Cl2, will be provided on <strong>the</strong> class web page<br />
for your use in <strong>the</strong> Data Analysis section <strong>of</strong> your lab report.<br />
How to Heat a Reaction with a Sand Bath/Magnetic Stirrer:<br />
In this experiment, both syn<strong>the</strong>ses require that you heat your reaction mixtures to boiling (or<br />
to "reflux") in a sand bath placed on top <strong>of</strong> a magnetic stirring plate. Getting <strong>the</strong> reaction mixture to<br />
stir well while heating can be problematic, so use <strong>the</strong> following procedure:<br />
4


![Experiment 9 Research Into Alternate Syntheses of [Cr(en)3]Cl3 ...](https://img.yumpu.com/18756539/1/190x245/experiment-9-research-into-alternate-syntheses-of-cren3cl3-.jpg?quality=85)