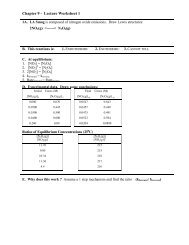
Experiment 4 Studying the Spectrochemical Series: Crystal Fields of ...
Experiment 4 Studying the Spectrochemical Series: Crystal Fields of ...
Experiment 4 Studying the Spectrochemical Series: Crystal Fields of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Experiment</strong> 4<br />
<strong>Studying</strong> <strong>the</strong> <strong>Spectrochemical</strong> <strong>Series</strong>: <strong>Crystal</strong> <strong>Fields</strong> <strong>of</strong> Cr(III)<br />
Introduction<br />
Coordination compounds <strong>of</strong> transition metals are <strong>of</strong>ten highly colored. The color results<br />
from absorption <strong>of</strong> light at specific wavelengths <strong>of</strong> visible light associated with electronic<br />
transitions within <strong>the</strong> d-orbitals. Thus, <strong>the</strong>se d-d transitions give many transition metal ions <strong>the</strong>ir<br />
characteristic color (eg: cobalt blue).<br />
The d orbitals <strong>of</strong> a metal ion in an octahedral crystal field (surrounded by an octahedral<br />
array <strong>of</strong> ligands) are split into a higher energy eg set and a lower energy t2g set (Figure 1). This is<br />
due to electron clouds around each ligand (L) destabilizing those d-orbitals that lie along <strong>the</strong> X, Y,<br />
and Z axes. The energy difference between <strong>the</strong> upper and lower energy levels is designated as Δo<br />
(pronounced del-oh) or 10Dq.<br />
Figure 1. d-orbitals split by an octahedral crystal field.<br />
The degree <strong>of</strong> splitting <strong>of</strong> <strong>the</strong> d orbitals and hence <strong>the</strong> magnitude <strong>of</strong> Δo depends on several<br />
factors, but <strong>the</strong> most important are <strong>the</strong> charge on <strong>the</strong> metal and <strong>the</strong> identity <strong>of</strong> <strong>the</strong> ligand.<br />
1
Understanding <strong>the</strong> trends in ligand-field splitting is simplified considerably by considering a series<br />
<strong>of</strong> complexes with <strong>the</strong> same metal in a given oxidation state; <strong>the</strong> only major variable in this case is<br />
<strong>the</strong> ligand identity. From a large number <strong>of</strong> studies it is known that ligands can be arranged in a<br />
sequence according to <strong>the</strong>ir ability to cause d-orbital splitting. This series is known as <strong>the</strong><br />
spectrochemical series:<br />
halides < NCS¯ < OH¯ < oxalate < H2O < NCS¯ < pyridine < NH3 < en < NO2¯ <<br />
CN¯ < CO<br />
(Underlined atom is <strong>the</strong> one coordinated to <strong>the</strong> metal).<br />
Note <strong>the</strong> general trend shown here: halide ligands are weaker-field ligands than sulfur<br />
donors, which are weaker field than oxygen donor ligands, which are weaker-field than nitrogen<br />
donors, which are weaker-field than multiply-bonded ligands. The stronger <strong>the</strong> interaction <strong>of</strong> <strong>the</strong><br />
ligand with <strong>the</strong> metal's d-orbitals, <strong>the</strong> stronger <strong>the</strong> field strength <strong>of</strong> that ligand.<br />
The magnitude <strong>of</strong> ligand field strength increases by a factor <strong>of</strong> about two as one moves from<br />
halide to CN¯ in <strong>the</strong> spectrochemical series.<br />
The structure <strong>of</strong> <strong>the</strong> oxalate ion, C2O4 2- , is shown below. Note <strong>the</strong> resonance forms <strong>of</strong> this<br />
compound.<br />
O<br />
O<br />
C C<br />
-O O- Figure 2. Structure <strong>of</strong> <strong>the</strong> oxalate ion<br />
- O<br />
O<br />
C C<br />
O O- - O<br />
O<br />
C<br />
- O<br />
C<br />
O<br />
2
The objective <strong>of</strong> this experiment is to quantify Δo for a series <strong>of</strong> Cr(III) complexes by<br />
electronic absorption spectroscopy. Cr(III) compounds are d 3 , and <strong>the</strong>ir electronic spectral<br />
characteristics are reasonably easy to interpret.<br />
Because <strong>of</strong> electronic selection rules (see lecture), <strong>the</strong> absorption band corresponding to <strong>the</strong><br />
energy <strong>of</strong> <strong>the</strong> crystal field strength (Δo) will be <strong>the</strong> one at <strong>the</strong> longest wavelength (lowest energy) in<br />
<strong>the</strong> spectrum, and it should be more intense than any o<strong>the</strong>r nearby transition.<br />
Ordering <strong>the</strong> octahedral Cr III compounds from longest to shortest wavelength will place <strong>the</strong><br />
ligands in order <strong>of</strong> increasing crystal field strength, as # " 1 ! o , and will allow you to build your<br />
own spectrochemical series.<br />
In mixed-ligand complexes, <strong>the</strong> “Rule <strong>of</strong> Average Environments” states that <strong>the</strong> observed<br />
value <strong>of</strong> Δo in such complexes is <strong>the</strong> weighted average <strong>of</strong> Δo for each <strong>of</strong> <strong>the</strong> homoleptic (single type<br />
<strong>of</strong> ligand) complexes. The first equation below is general, <strong>the</strong> second equation is for <strong>the</strong> specific<br />
example <strong>of</strong> [Cr(H2O)4Cl2] + .<br />
If <strong>the</strong> Δo's <strong>of</strong> [Cr(H2O)4Cl2] + and [Cr(H2O)6] 3+ are known, <strong>the</strong>n by rearranging <strong>the</strong> equation,<br />
you can solve for <strong>the</strong> Δo <strong>of</strong> [CrCl6] 3+ . You can use this Δo to find <strong>the</strong> Δo <strong>of</strong> o<strong>the</strong>r unknown<br />
complexes.<br />
Syn<strong>the</strong>ses<br />
ΔoMAnBm = (1/6) {nΔoMA6 + mΔoMB6} (1)<br />
Δo[Cr(H2O)4Cl2] + = (1/6) {4Δo[Cr(H2O)6] 3+ + 2Δo[CrCl6] 3- } (2)<br />
In this experiment, <strong>the</strong> bidentate ligand acetylacetonate (acac¯) will be generated via <strong>the</strong><br />
deprotonation <strong>of</strong> acetylacetone (acacH) by ammonia. The ammonia is generated by hydrolysis <strong>of</strong><br />
urea (Figure 3); subsequently, ammonia acts as a base to deprotonate acacH. Note <strong>the</strong> resonance<br />
forms <strong>of</strong> acac-.<br />
3
O<br />
H 2N NH 2<br />
O<br />
H3C C CH3 H2 + H 2O 2 NH 3 + CO 2<br />
O<br />
H<br />
O<br />
O<br />
H3C C CH3 H<br />
O<br />
O<br />
H3C C CH3 H<br />
Figure 3. Top: Hydrolysis <strong>of</strong> urea. Bottom: Deprotonation <strong>of</strong> acetylacetone forms <strong>the</strong> bidentate<br />
ligand acetylacetonate (acac-). Systematic name is 2,4-pentanedionate.<br />
<strong>Experiment</strong>al Procedure<br />
You will work in pairs in this lab to prepare two compounds: Cr(acac)3, and<br />
[Cr(en)3]Cl3•2H2O. One <strong>of</strong> <strong>the</strong> pair will syn<strong>the</strong>size one compound, and <strong>the</strong> o<strong>the</strong>r partner will<br />
prepare <strong>the</strong> o<strong>the</strong>r compound. The procedure for <strong>the</strong> syn<strong>the</strong>ses for *both* compounds must be<br />
written in your prelab. You will <strong>the</strong>n share experimental and spectroscopic data (for all <strong>the</strong><br />
compounds, not just for <strong>the</strong> two that you prepare).<br />
Ano<strong>the</strong>r compound, [Cr(H2O)6](NO3)3•3H2O, will be provided to you; you will<br />
spectroscopically analyze both this complex and your starting material, CrCl3•6H2O (this compound<br />
is actually [CrCl2(H2O)4]Cl•2H2O]).<br />
Finally, <strong>the</strong> electronic spectrum <strong>of</strong> [Cr(NH3)5Cl]Cl2, will be provided on <strong>the</strong> class web page<br />
for your use in <strong>the</strong> Data Analysis section <strong>of</strong> your lab report.<br />
How to Heat a Reaction with a Sand Bath/Magnetic Stirrer:<br />
In this experiment, both syn<strong>the</strong>ses require that you heat your reaction mixtures to boiling (or<br />
to "reflux") in a sand bath placed on top <strong>of</strong> a magnetic stirring plate. Getting <strong>the</strong> reaction mixture to<br />
stir well while heating can be problematic, so use <strong>the</strong> following procedure:<br />
4
Place an empty beaker containing a stir bar on <strong>the</strong> magnetic stirrer, and turn on <strong>the</strong> stirrer.<br />
Check to see that <strong>the</strong> bar can stir quickly and well. If it cannot, use a different magnetic stirrer.<br />
Place your sand bath on top <strong>of</strong> <strong>the</strong> magnetic stirrer and bury your flask containing <strong>the</strong> stir<br />
bar and <strong>the</strong> solution to be heated deep into <strong>the</strong> sand. Turn on <strong>the</strong> magnetic stirrer very slowly, and<br />
try to get <strong>the</strong> stir bar to stir vigorously. There is <strong>of</strong>ten a problem in accomplishing this, because <strong>the</strong><br />
stir bar is too far away from <strong>the</strong> magnetic stirrer to respond well to it. To correct this, take sand out<br />
<strong>of</strong> your sand bath (place it in a beaker and RETURN IT to <strong>the</strong> sand bath when you are finished)<br />
and move your flask down towards <strong>the</strong> bottom <strong>of</strong> <strong>the</strong> bath, until it almost touches <strong>the</strong> metal bottom<br />
<strong>of</strong> <strong>the</strong> sand bath. The closer <strong>the</strong> bottom <strong>of</strong> <strong>the</strong> flask comes to <strong>the</strong> bottom <strong>of</strong> <strong>the</strong> sand bath, <strong>the</strong> better<br />
<strong>the</strong> stir bar will stir. Do not however, let <strong>the</strong> bottom <strong>of</strong> your flask come into direct contact with <strong>the</strong><br />
metal <strong>of</strong> <strong>the</strong> sand bath; <strong>the</strong> flask may melt.<br />
Again, turn on <strong>the</strong> magnetic stirrer very slowly, and keep removing sand and moving <strong>the</strong><br />
flask down into <strong>the</strong> bath until <strong>the</strong> bar is stirring vigorously and well.<br />
Now heap <strong>the</strong> extra sand back into <strong>the</strong> bath underneath and around <strong>the</strong> shoulders <strong>of</strong> <strong>the</strong><br />
flask, to help transfer heat and encourage quick heating <strong>of</strong> <strong>the</strong> solution. When your reaction<br />
mixture is vigorously stirring, and <strong>the</strong> extra sand has been replaced around <strong>the</strong> shoulders <strong>of</strong> <strong>the</strong><br />
flask, turn on <strong>the</strong> sand bath and heat your reaction mixture.<br />
Make sure, if possible, that your reaction mixture is stirring vigorously throughout <strong>the</strong><br />
heating period. If <strong>the</strong> stirring stops, repeat <strong>the</strong> above procedure until you can get efficient stirring<br />
again.<br />
A. Cr(acac)3 – <strong>the</strong> preparation <strong>of</strong> Tris(2,4-pentanedionate)chromium(III)<br />
Dissolve 260 mg <strong>of</strong> CrCl3•6H2O in 4.0 mL <strong>of</strong> distilled water in a 25 mL round-bottomed<br />
flask, and <strong>the</strong>n add 1 g <strong>of</strong> urea to <strong>the</strong> flask. Measure out 0.8 mL <strong>of</strong> acetylacetone using <strong>the</strong> 1-mL<br />
graduated pipette provided, and add <strong>the</strong> acetylacetone to <strong>the</strong> flask also. Next place a reflux<br />
condenser on <strong>the</strong> flask, after first applying a thin layer <strong>of</strong> stopcock grease to <strong>the</strong> male ground glass<br />
joint <strong>of</strong> <strong>the</strong> reflux condenser. Reflux your reaction, with stirring, for one hour using a sand bath. As<br />
<strong>the</strong> urea releases ammonia and <strong>the</strong> solution becomes basic, deep maroon crystals begin to form.<br />
5
After one hour, cool <strong>the</strong> flask thoroughly in an ice bath. Very thorough cooling is needed. If<br />
no crystals form, carefully make a small scratch on <strong>the</strong> bottom <strong>of</strong> <strong>the</strong> flask using a glass rod or<br />
metal spatula, and cool <strong>the</strong> flask again. Collect <strong>the</strong> crystals by suction filtration and wash <strong>the</strong>m with<br />
three 0.3 mL portions <strong>of</strong> distilled water. Dry <strong>the</strong> crystals as much as possible on <strong>the</strong> filter using <strong>the</strong><br />
aspirator, and <strong>the</strong>n collect <strong>the</strong>m and spread <strong>the</strong>m on a piece <strong>of</strong> filter paper to air dry. Determine <strong>the</strong><br />
percentage yield, and transfer <strong>the</strong> Cr(acac)3 to a labeled vial.<br />
B. [Cr(en)3]Cl3•2H2O – <strong>the</strong> preparation <strong>of</strong> Tris(ethylenediamine)chromium(III)<br />
Weigh out 100 mg <strong>of</strong> mossy zinc into a 25 mL round-bottomed flask. Remove <strong>the</strong> surface<br />
layer <strong>of</strong> ZnO and generate a clean and active Zn 0 surface by washing <strong>the</strong> Zn with HCl immediately<br />
prior to use. To accomplish this, add enough 6M HCl to cover <strong>the</strong> mossy zinc, swirl <strong>the</strong> flask briefly<br />
(<strong>the</strong> mossy zinc may completely dissolve upon prolonged contact with HCl), and pipette <strong>of</strong>f <strong>the</strong><br />
acid. Then wash <strong>the</strong> mossy zinc three times with 10 mL portions <strong>of</strong> methanol, to remove as much<br />
acid as possible.<br />
Weigh out 266 mg <strong>of</strong> CrCl3•6H2O, and add it and 1 mL <strong>of</strong> methanol to <strong>the</strong> round-bottomed<br />
flask. In <strong>the</strong> hood, add 1 mL <strong>of</strong> ethylenediamine. Next place a reflux condenser on <strong>the</strong> flask, after<br />
first applying a thin layer <strong>of</strong> stopcock grease to <strong>the</strong> male ground glass joint <strong>of</strong> <strong>the</strong> reflux condenser.<br />
Reflux your reaction, with stirring, for one hour using a sand bath.<br />
Take care not to turn up <strong>the</strong> sand bath power too high: methanol refluxes at a lower<br />
temperature (65 o C) than water (100 o C).<br />
Cool <strong>the</strong> purple solution in an ice bath. Collect <strong>the</strong> yellow crystalline product (it will appear<br />
purple, because it is wet with <strong>the</strong> solution) by suction filtration using a Hirsch funnel. Remove any<br />
un-reacted zinc with tweezers.<br />
Wash <strong>the</strong> filtered product with 0.5 mL portions <strong>of</strong> 10% ethylenediamine in methanol until<br />
<strong>the</strong> purple solution is gone, <strong>the</strong> product is yellow, and <strong>the</strong> washings are colorless. Follow this with a<br />
0.5 mL rinse with e<strong>the</strong>r, to rinse away <strong>the</strong> methanol and help dry <strong>the</strong> product. Dry <strong>the</strong> crystals as<br />
much as possible on <strong>the</strong> filter using <strong>the</strong> aspirator, and <strong>the</strong>n collect <strong>the</strong>m and spread <strong>the</strong>m on a piece<br />
<strong>of</strong> filter paper to air dry. Determine <strong>the</strong> percentage yield, and transfer <strong>the</strong> [Cr(en)3]Cl3•2H2O to a<br />
labeled vial.<br />
6
C. Spectroscopy <strong>of</strong> <strong>the</strong> Cr(III) Complexes.<br />
Prepare aqueous solutions <strong>of</strong> [Cr(en)3]Cl3•2H2O and [Cr(H2O)6](NO3)3•3H2O by dissolving<br />
approximately 8 mg <strong>of</strong> each complex in about 5 ml <strong>of</strong> distilled water. Because <strong>the</strong> analysis <strong>of</strong> Δo,<br />
<strong>the</strong> objective <strong>of</strong> this experiment, requires only that <strong>the</strong> longest-wavelength λmax <strong>of</strong> each complex be<br />
identified, you do not need to know <strong>the</strong> exact concentration <strong>of</strong> <strong>the</strong> solution, as you do when<br />
calculating extinction coefficients using Beer's law.<br />
Also prepare a similar ethanol solution <strong>of</strong> Cr(acac)3.<br />
Make a similar solution <strong>of</strong> CrCl3•6H2O in water, but note that this complex, consisting <strong>of</strong><br />
[CrCl2(H2O)4] + ions in water, slowly substitutes to <strong>the</strong> hexaaquo species, [Cr(H2O)6] 3+ +, so it should<br />
be analyzed immediately after its preparation. Do not prepare this solution until your name is called<br />
for use <strong>of</strong> <strong>the</strong> UV-vis instrument. At that point, quickly add already-prepared portions <strong>of</strong><br />
CrCl3•6H2O and water to a cuvette , mix <strong>the</strong> solution a few times by drawing it up in a Pasteur<br />
pipette, and <strong>the</strong>n acquire <strong>the</strong> spectrum <strong>of</strong> this solution first.<br />
Obtain <strong>the</strong> electronic absorption spectrum <strong>of</strong> each complex. Record <strong>the</strong> wavelengths and<br />
absorbance values for all absorbances for each sample in your notebook. Determine and record <strong>the</strong><br />
longest wavelength <strong>of</strong> <strong>the</strong> absorbance peaks for each complex in units <strong>of</strong> nanometers. This is <strong>the</strong><br />
wavelength you will use to calculate <strong>the</strong> Δo's <strong>of</strong> <strong>the</strong> complexes in <strong>the</strong> Data Analysis section <strong>of</strong> your<br />
lab report.<br />
Data Analysis<br />
1. (3 points). Draw <strong>the</strong> crystal field energy levels and electron occupancies for octahedral Cr 3+ ions.<br />
Indicate <strong>the</strong> energy gap that corresponds to <strong>the</strong> transition that is being investigated in this lab.<br />
2. (10 points) Prepare a table <strong>of</strong> your data, including columns for λmax in nm and ΔO in cm -1 , eV, and<br />
kJ/mol.<br />
a) Convert <strong>the</strong> wavelengths which correspond to Δo into wavenumbers (cm -1 ) using <strong>the</strong><br />
7
following relationship:<br />
Δo = [1/λ (nm)] (1 x 10 7 ) cm -1<br />
b) O<strong>the</strong>r energy units for Δo may be obtained using <strong>the</strong> following conversion factors:<br />
1 cm -1 = 1.24 x 10 -4 eV = 0.01196 kJ/mol<br />
c) Arrange <strong>the</strong> entries in order <strong>of</strong> increasing gap energy.<br />
Show your complete set <strong>of</strong> calculations for one <strong>of</strong> <strong>the</strong> complexes. For <strong>the</strong> rest <strong>of</strong> <strong>the</strong><br />
complexes, just record <strong>the</strong> calculated values in <strong>the</strong> table.<br />
3. (12 points) List <strong>the</strong> five ligands in order <strong>of</strong> increasing ligand field strength.<br />
Use <strong>the</strong> Rule <strong>of</strong> Average Environments to calculate <strong>the</strong> true ligand field strengths <strong>of</strong> two <strong>of</strong><br />
<strong>the</strong> ligands (to be included in <strong>the</strong> list above). Show your complete set <strong>of</strong> calculations.<br />
Discussion<br />
1. (10 points) Discuss <strong>the</strong> order <strong>of</strong> <strong>the</strong> ligand field strengths you obtained, comparing <strong>the</strong>m with <strong>the</strong><br />
spectrochemical series given in <strong>the</strong> introduction. Does <strong>the</strong> order <strong>of</strong> ligands obtained by this<br />
experiment correspond to <strong>the</strong> established order <strong>of</strong> <strong>the</strong> spectrochemical series? Explain any<br />
deviations.<br />
2. (5 points) List <strong>the</strong> five ligands in order <strong>of</strong> <strong>the</strong> increasing strength <strong>of</strong> <strong>the</strong>ir interaction with Cr +3 ,<br />
and give <strong>the</strong> reasoning behind your answer.<br />
3. (5 points) The ligand acac¯ is not included in <strong>the</strong> spectrochemical series given in <strong>the</strong> Introduction.<br />
Examining <strong>the</strong> chemical structure <strong>of</strong> acac - and <strong>of</strong> those <strong>of</strong> related ligands shown in <strong>the</strong><br />
spectrochemical series, explain where you would expect to find acac - in <strong>the</strong> spectrochemical series.<br />
8
Does your calculated ligand field strength for acac - agree with this prediction?<br />
Conclusions (10 points): Discuss what knowledge has been gained by this experiment. Discuss <strong>the</strong><br />
importance to inorganic chemistry <strong>of</strong> being able to ascertain <strong>the</strong> crystal field energy gap <strong>of</strong><br />
complexes, and <strong>the</strong> ligand field strength <strong>of</strong> ligands. What information can be obtained, or what<br />
advantages in o<strong>the</strong>r experiments can be gained, about inorganic complexes using <strong>the</strong>se two<br />
quantities?<br />
Questions<br />
1. (5 points) What is a significant difference that is seen between <strong>the</strong> UV-visible spectrum <strong>of</strong><br />
Cr(acac)3 and <strong>the</strong> spectra <strong>of</strong> <strong>the</strong> o<strong>the</strong>r complexes? What is <strong>the</strong> reason for this difference?<br />
2. (5 points) High-spin Mn(II) and Fe(III) complexes are much less intensely colored than those <strong>of</strong><br />
Cr(III). Why are <strong>the</strong>y so weakly colored?<br />
9



![Experiment 9 Research Into Alternate Syntheses of [Cr(en)3]Cl3 ...](https://img.yumpu.com/18756539/1/190x245/experiment-9-research-into-alternate-syntheses-of-cren3cl3-.jpg?quality=85)