SnapShot: Hedgehog Signaling Pathway - Cardiovascular ...
SnapShot: Hedgehog Signaling Pathway - Cardiovascular ...
SnapShot: Hedgehog Signaling Pathway - Cardiovascular ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
386 Cell 130, July 27, 2007 ©2007 Elsevier Inc. DOI 10.1016/j.cell.2007.07.017 See online version for legend, abbreviations, and references.<br />
<strong>SnapShot</strong>: <strong>Hedgehog</strong> <strong>Signaling</strong> <strong>Pathway</strong><br />
Miao-Hsueh Chen, Christopher W. Wilson, and Pao-Tien Chuang<br />
<strong>Cardiovascular</strong> Research Institute, University of California, San Francisco, CA 94158, USA
<strong>SnapShot</strong>: <strong>Hedgehog</strong><br />
<strong>Signaling</strong> <strong>Pathway</strong><br />
Miao-Hsueh Chen, Christopher W. Wilson, and Pao-Tien Chuang<br />
<strong>Cardiovascular</strong> Research Institute, University of California, San Francisco, CA 94158, USA<br />
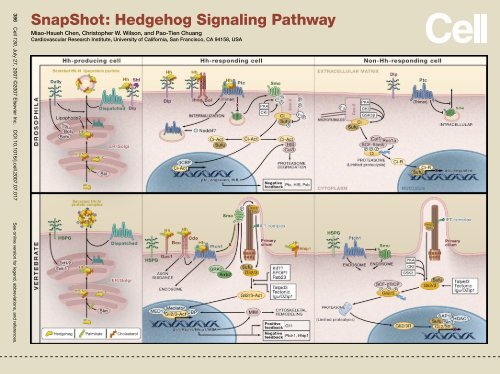
The <strong>Hedgehog</strong> (Hh) signal transduction pathway controls numerous processes during fly and vertebrate embryonic development and<br />
adult homeostasis, including tissue/organ patterning, cellular proliferation and differentiation, pathfinding, left/right asymmetry, and<br />
stem cell maintenance. Hh signaling is dysregulated in several congenital defects and many types of tumors. Hh is able to signal at<br />
short-range but also may act as a long-range morphogen in multiple contexts (this aspect is controlled in part by lipid modification of<br />
the Hh molecule). Hh signaling controls the balance of the transcriptional activator and repressor forms of Ci/Gli, the ratios of which are<br />
essential for interpreting the level of Hh signal in a morphogenetic field and for generating diverse outputs.<br />
Hh-Producing Cell<br />
After translation, the Hh precursor enters the ER/Golgi secretory pathway. Autoproteolysis mediated by the C-terminal of Hh (Hh-C)<br />
releases the N-terminal signaling peptide (Hh-N), with cholesterol covalently linked to its C terminus. Addition of a palmitate moiety to<br />
the N terminus of the Hh signaling fragment is catalyzed by Skinny hedgehog (Ski/Skn). Lipid modifications are essential for the Hh<br />
ligand to induce high-level signaling activity and proper formation of a morphogen gradient. Lipidated Hh is assisted in its release from<br />
cells by Dispatched and possibly by the heparan sulfate proteoglycans (HSPGs) Dally and Dally-like (Dlp) and can be detected in distant<br />
Hh-responsive cells. Although the mechanism of lipidated Hh-N release into the extracellular matrix is unknown, two soluble forms<br />
of lipidated Hh-N have been detected including a multimeric protein complex in both flies and vertebrates and a lipoprotein particle<br />
in flies. In flies, Hh in the extracellular matrix is protected from degradation by Shifted (Shf) and is funneled to its destination by Dlp.<br />
Specific HSPG core proteins may play a role in vertebrate Hh signaling, but this has not yet been shown.<br />
Hh-Responding Cell<br />
Hh elicits transcriptional responses by binding to its trimeric receptor Patched (Ptc/Ptch1). Additional Hh coreceptors (Ihog family<br />
members, HSPGs, and Gas1) may participate in induction of both transcription-dependent and -independent responses. Binding of<br />
Hh to Ptc alleviates repression of Smoothened (Smo), allowing its translocation from endosomes to the cell surface in flies or to the<br />
primary cilium in mice. Inhibition of Ptc results in phosphorylation of the cytosolic C-tail of Smo. Flies and vertebrates may use different<br />
strategies downstream of Smo to modulate activity of the Ci/Gli transcription factors. In fly, recruitment of the atypical kinesin<br />
Costal-2 (Cos-2)/Fused kinase (Fu)/Cubitus interruptus (Ci) complex to the phosphorylated Smo C-tail results in activation of the Ci<br />
transcription factor, which then enters the nucleus and activates target gene expression. In vertebrates, generation of activated Gli<br />
transcription factors involves the primary cilium, but it is currently unclear if a kinesin-like scaffold is an intermediary between Smo<br />
and Gli. Numerous poorly characterized factors have been reported to play roles in modulating Gli activator and repressor activity. Two<br />
general mechanisms exist to downregulate Hh signaling after the initial response. Ci/Gli transcription factors are ubiquitinated by Cullin<br />
(Cul3) and HIB/SPOP complexes; the expression of Hh-binding proteins, such as Ptc and Hhip1, is upregulated to serve as a sink for<br />
extracellular ligand.<br />
Hh-Nonresponding Cell<br />
In the absence of Hh ligand, Ptc inhibits Smo and prevents its translocation to the cell surface in flies or to the primary cilium in mice.<br />
The nucleocytoplasmic distribution of newly synthesized Ci is controlled by the Ci/Gli-binding protein Sufu. In fly, microtubule-associated<br />
Cos-2 serves as a scaffold for protein kinase A (PKA), casein kinase I (CKI), and glycogen synthase kinase 3 (GSK3), which phosphorylate<br />
Ci. The SCF-Slimb-Cul1-Roc1a complex recognizes phosphorylated Ci and targets it for proteasome-dependent limited proteolysis,<br />
which removes C-terminal transcriptional activation domains. The newly generated Ci repressor translocates to the nucleus<br />
where it inhibits Hh target gene activation in conjunction with Sufu. In vertebrates, the primary cilium is involved in efficient generation<br />
of Gli repressors, which are formed through a similar phosphorylation-dependent mechanism. Sufu may also mediate interactions<br />
between Gli repressors and the HDAC1 repression complex.<br />
Abbreviations<br />
Arrb2, arrestin β2; Boc, cdo-binding protein; Boi, brother of ihog; Botv, brother of tout-velu; Cdo, cell adhesion molecule-related/downregulated<br />
by oncogenes; Ext1/2, exostoses 1/2; Extl3, exostoses-like 3; Gas1, growth arrest specific-1; GRK2, G protein-coupled receptor kinase<br />
2; HDAC1, histone deacetylase 1 complex; Ihog, interference hedgehog; IFT, intraflagellar transport; Igu/DZip1, iguana/DAZ interacting protein<br />
1; MIM, missing in metastasis; Sotv, sister of tout-velu; SPOP, speckle-type POZ protein; Sufu, suppressor of fused; Ttv, tout-velu.<br />
RefeRences<br />
Davey, M.G., Paton, I.R., Yin, Y., Schmidt, M., Bangs, F.K., Morrice, D.R., Smith, T.G., Buxton, P., Stamataki, D., Tanaka, M., et al. (2006). The chicken talpid3 gene<br />
encodes a novel protein essential for <strong>Hedgehog</strong> signaling. Genes Dev. 20, 1365–1377.<br />
Eaton, S. (2006). Release and trafficking of lipid-linked morphogens. Curr. Opin. Genet. Dev. 16, 17–22.<br />
Fuccillo, M., Joyner, A.L., and Fishell, G. (2006). Morphogen to mitogen: The multiple roles of hedgehog signalling in vertebrate neural development. Nat. Rev. Neurosci.<br />
7, 772–783.<br />
Gonzalez-Quevedo, R., Shoffer, M., Horng, L., and Oro, A.E. (2005). Receptor tyrosine phosphatase-dependent cytoskeletal remodeling by the hedgehog-responsive<br />
gene MIM/BEG4. J. Cell Biol. 168, 453–463.<br />
386.e1 Cell 130, July 27, 2007 ©2007 Elsevier Inc. DOI 10.1016/j.cell.2007.07.017
<strong>SnapShot</strong>: <strong>Hedgehog</strong><br />
<strong>Signaling</strong> <strong>Pathway</strong><br />
Miao-Hsueh Chen, Christopher W. Wilson, and Pao-Tien Chuang<br />
<strong>Cardiovascular</strong> Research Institute, University of California, San Francisco, CA 94158, USA<br />
Guerrero, I., and Chiang, C. (2007). A conserved mechanism of <strong>Hedgehog</strong> gradient formation by lipid modifications. Trends Cell Biol. 17, 1–5.<br />
Hooper, J.E., and Scott, M.P. (2005). Communicating with <strong>Hedgehog</strong>s. Nat. Rev. Mol. Cell Biol. 6, 306–317.<br />
Huangfu, D., and Anderson, K.V. (2006). <strong>Signaling</strong> from Smo to Ci/Gli: Conservation and divergence of <strong>Hedgehog</strong> pathways from Drosophila to vertebrates. Development<br />
133, 3–14.<br />
Incardona, J.P., Gruenberg, J., and Roelink, H. (2002). Sonic hedgehog induces the segregation of patched and smoothened in endosomes. Curr. Biol. 12, 983–995.<br />
Ingham, P.W., and McMahon, A.P. (2001). <strong>Hedgehog</strong> signaling in animal development: Paradigms and principles. Genes Dev. 15, 3059–3087.<br />
Jia, J., and Jiang, J. (2006). Decoding the <strong>Hedgehog</strong> signal in animal development. Cell. Mol. Life Sci. 63, 1249–1265.<br />
Kalderon, D. (2005). The mechanism of hedgehog signal transduction. Biochem. Soc. Trans. 33, 1509–1512.<br />
Lu, X., Liu, S., and Kornberg, T.B. (2006). The C-terminal tail of the <strong>Hedgehog</strong> receptor Patched regulates both localization and turnover. Genes Dev. 20, 2539–2551.<br />
Mann, R.K., and Beachy, P.A. (2004). Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 73, 891–923.<br />
Nieuwenhuis, E., and Hui, C.C. (2005). <strong>Hedgehog</strong> signaling and congenital malformations. Clin. Genet. 67, 193–208.<br />
Ogden, S.K., Ascano, M., Jr., Stegman, M.A., and Robbins, D.J. (2004). Regulation of <strong>Hedgehog</strong> signaling: A complex story. Biochem. Pharmacol. 67, 805–814.<br />
Pan, Y., Bai, C.B., Joyner, A.L., and Wang, B. (2006). Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation.<br />
Mol. Cell. Biol. 26, 3365–3377.<br />
Singla, V., and Reiter, J.F. (2006). The primary cilium as the cell’s antenna: <strong>Signaling</strong> at a sensory organelle. Science 313, 629–633.<br />
Varjosalo, M., Li, S.P., and Taipale, J. (2006). Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev. Cell 10, 177–186.<br />
Wilson, C.W., and Chuang, P.-T. (2006). New “hogs” in <strong>Hedgehog</strong> transport and signal reception. Cell 125, 435–438.<br />
386.e2 Cell 130, July 27, 2007 ©2007 Elsevier Inc. DOI 10.1016/j.cell.2007.07.017