Avicel DG Binder - FMC BioPolymer
Avicel DG Binder - FMC BioPolymer
Avicel DG Binder - FMC BioPolymer
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>FMC</strong> <strong>BioPolymer</strong><br />
<strong>Avicel</strong> ®<br />
<strong>DG</strong> <strong>Binder</strong><br />
A Superior Excipient for<br />
Roller Compaction and Dry Granulation
Introduction: Dry Granulation <strong>Binder</strong><br />
The use of dry granulation production techniques, like roller<br />
compaction, continue to develop in the pharmaceutical<br />
industry. It is an economical way to achieve good flow and<br />
content uniformity. It has significant advantages over wet<br />
granulation, primarily due to the elimination of the drying<br />
step.<br />
Advantages of this type of dry granulation process include:<br />
• High-volume production of granules<br />
• Uniform particle size<br />
• Suitability for moisture and heat sensitive active<br />
pharmaceutical ingredients (API)<br />
• Good control of bulk density and flow<br />
• Consistency of the final dosage form<br />
• Consistent blend of excipients and API<br />
Product Description: <strong>Avicel</strong> ® <strong>DG</strong><br />
<strong>Avicel</strong> <strong>DG</strong> is a high functionality excipient optimized for use in dry granulation processes. <strong>Avicel</strong> <strong>DG</strong> is the<br />
result of a synergistic combination of two pharmaceutical excipients produced using <strong>FMC</strong>’s expertise in<br />
spray dried, co-processing technology.<br />
<strong>Avicel</strong> <strong>DG</strong> occurs as a white, odorless powder containing 75% of microcrystalline<br />
cellulose* and 25% anhydrous dibasic calcium phosphate*. The wet<br />
dispersion and spray drying of microcrystalline cellulose and anhydrous dibasic<br />
calcium phosphate results in an intimate physical combination, which cannot<br />
be achieved by traditional dry blending.<br />
The unique characteristics of <strong>Avicel</strong> <strong>DG</strong> facilitate solid dosage form<br />
manufacturing utilizing roller compaction by improving process<br />
robustness, tablet hardness, and yields.<br />
As an example, a model formulation containing Vitamin C (Ascorbic Acid),<br />
lubricant and <strong>Avicel</strong> <strong>DG</strong>, compacted at 30 bars, produced tablet tensile<br />
strengths 100-175% better than formulations containing physical blends<br />
of common excipients.<br />
* Both microcrystalline cellulose and anhydrous dibasic calcium phosphate are listed as<br />
separate monographs in the JP, Ph Eur, and USP-NF, but the combination is not listed.<br />
Scanning Electron Micrographs<br />
(1000 x)<br />
Microcrystalline cellulose<br />
<strong>Avicel</strong> ® <strong>DG</strong>
Advantages of <strong>Avicel</strong> <strong>DG</strong> <strong>Binder</strong><br />
The use of <strong>Avicel</strong> <strong>DG</strong> improves yields by dramatically enhancing recompactability. It reduces costs<br />
by minimizing steps in the production process.<br />
<strong>Avicel</strong> <strong>DG</strong> offers good flow characteristics, a high quality initial compactability, required for the<br />
production of robust ribbons, and excellent recompactability, resulting in enhanced tabletting<br />
performance and fewer rejected tablets.<br />
These features will result in better yields and savings:<br />
• Increase of tablet hardness<br />
• Reduction of equipment wear enabled by lower tabletting forces<br />
• Elimination of time consuming weighing and blending steps<br />
• Reduction of rejects due to improved tablet quality<br />
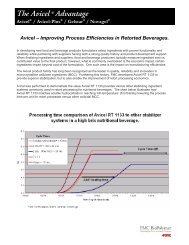
<strong>Avicel</strong> <strong>DG</strong> doubles tablet strength as compared to physical blends<br />
Tablet tensile strength at 120 MPa tabletting pressure, after compaction at 30 bars.Model formulation: 69.5% excipient, 30% Vitamin C<br />
(ascorbic acid), 0.5% magnesium stearate.
<strong>Avicel</strong> <strong>DG</strong> binder extracts the full value from your roller compaction process<br />
Cost savings are driven by the reduction of labor-associated steps within the process and improved<br />
tablet quality.<br />
• Raw materials - reduced number of excipients, inventory, QC and handling<br />
• Blending - reduced dispensing, weighing and handling<br />
• Roller compaction - improved ribbon robustness<br />
• Extragranular binder - can be eliminated<br />
• Tabletting - reduction of required tabletting forces and resulting equipment wear<br />
• Tablet control - improved yields due to enhanced recompactability<br />
• Coating and packaging - reduced rejects due to lower tablet friability<br />
Poorly flowing blends are compacted and milled into uniform granules with good flow<br />
If the elimination of the post granulation blending steps can be validated, resulting in the absence<br />
of extragranular disintegrant and lubricant, roller compaction may evolve into a truly continuous<br />
production process with consistent performance and stability.
<strong>Avicel</strong> <strong>DG</strong> significantly improves tablet robustness at all tabletting pressure<br />
Tablet tensile strength at increasing tabletting pressures, after a compaction at 30 bars. Model formulation: 69.5% excipient, 30% Vitamin C<br />
(ascorbic acid), 0.5% magnesium stearate.<br />
<strong>Avicel</strong> <strong>DG</strong> demonstrates a clear improvement over physical blends of excipients<br />
<strong>Avicel</strong> <strong>DG</strong> <strong>Binder</strong> exhibits enhanced recompactability and higher tensile strength compared to either<br />
MCC or the physical blends of MCC and lactose, or MCC and dicalcium phosphate.<br />
In this model formulation, physical blends, without the addition of an extragranular excipient, offered<br />
a maximum tensile strength of about 1.0 MPa. This is typical of weak and friable tablets. <strong>Avicel</strong> <strong>DG</strong>,<br />
without any additional excipients, enabled the production of robust tablets, characterized with tensile<br />
strengths exceeding 2.0 MPa.<br />
The use of <strong>Avicel</strong> <strong>DG</strong> may enable the use of roller compaction for formulations which previously could<br />
not achive adequate tablet strength from a dry granulation process.
Compendial Status<br />
<strong>Avicel</strong> <strong>DG</strong> is co-processed in a cGMP production facility from two compendial excipients at a<br />
constant ratio to provide a quality high functionality excipient compatible with the majority of active<br />
pharmaceutical ingredients. It is processed and tested under tight controls to meet our high quality<br />
standards.<br />
Product Specifications<br />
<strong>Avicel</strong> <strong>DG</strong> (Dry Granulation <strong>Binder</strong>)<br />
[75% Microcrystalline Cellulose NF, Ph. Eur., JP]<br />
[25% Anhydrous Dibasic Calcium Phosphate USP, FCC, Ph. Eur., JP]<br />
Test Specfication<br />
Loss on drying, % NMT 5.0<br />
pH 5.5 - 7.5<br />
Sieve fraction, %, 2 - 35<br />
Alpine +200 mesh<br />
PS Malvern d50, m 35 - 55<br />
LBD, g/cc 0.25 - 0.40<br />
Assay DCP, % 21 - 29<br />
ID test A, Ca 2+<br />
Pass<br />
ID test B, PO4 3-<br />
Pass<br />
ID test C, cellulose Pass<br />
ID test D, degree of polymerization Pass<br />
Heavy metals, % NMT 0.001<br />
Water soluble substances, mg/5g NMT 20.0<br />
Ether soluble substances, mg/10g NMT 5.0<br />
Ether soluble substances, % NMT 0.050<br />
Microbial Limits<br />
Total aerobic microbial, count/g NMT 1000<br />
Total yeast and mold, count/g NMT 100<br />
Pseudomonas aeruginosa None present in a 10g sample<br />
Escherichia coli None present in a 10g sample<br />
Staphylococcus aureus None present in a 10g sample<br />
Salmonella species None present in a 10g sample<br />
NMT = Not More Than
Contact <strong>FMC</strong> for assistance.<br />
<strong>FMC</strong> is the world leader in quality<br />
excipients for pharmaceuticals and<br />
supplements.<br />
As the world’s foremost manufacturer<br />
of high quality pharmaceutical excipients,<br />
<strong>FMC</strong> has vast experience in the areas<br />
of formulating and processing. When<br />
customers use <strong>Avicel</strong> they get the<br />
benefit of this unparalleled technical<br />
support.<br />
For further information on <strong>FMC</strong>’s<br />
outstanding products and services,<br />
contact us at 1800-526-3649, or visit<br />
us at our website at<br />
www.fmcbiopolymer.com
United States<br />
Philadelphia, Pennsylvania<br />
Sales/Technical<br />
Assistance:<br />
Tel: 1 215 299 6534<br />
Fax: 1 215 299 6669<br />
Customer Service:<br />
Tel: 1 800 526 3649<br />
Fax: 1 215 299 6475<br />
Europe<br />
Brussels, Belgium<br />
Sales/Technical<br />
Assistance:<br />
Tel: +32 2 775 8311<br />
Fax: +32 2 775 8300<br />
Customer Service:<br />
Tel: +353 21 4354 133<br />
Fax: +353 21 4517 201<br />
Patents<br />
<strong>FMC</strong> Corporation is owner and/or licensee of<br />
several patents related to its products. The<br />
products, processes and uses of such products<br />
referred to in this document may be covered by<br />
one or more patents or pending applications in<br />
the United States and/or other countries. <strong>FMC</strong><br />
does not warrant against any infringement claim<br />
arising from the sale and/or use of any <strong>FMC</strong><br />
product in combination with other materials;<br />
the use of any <strong>FMC</strong> product in the operation of<br />
any process; any <strong>FMC</strong> product manufactured to<br />
a customer's designs or specifications; or any<br />
<strong>FMC</strong> product manufactured by any process<br />
requested by a purchaser.<br />
www.fmcbiopolymer.com/pharmaceutical<br />
pharm_info@fmc.com<br />
The roller compaction equipment photos were provided<br />
by The Fitzpatrick Company, Elmhurst, Illinois<br />
<strong>FMC</strong> logo and <strong>Avicel</strong> are trademarks of <strong>FMC</strong>.<br />
© 2009 <strong>FMC</strong> Corporation.<br />
All rights reserved. 10/29/09.RS<br />
Asia-Pacific<br />
Hong Kong<br />
Tel: +852 2839 6600<br />
Fax: +852 2576 3770<br />
Tokyo, Japan<br />
Tel: +81 3 3402 3739<br />
Fax: +81 3 3402 3700<br />
Product Suitability<br />
The information contained in this document<br />
(as well as any advice or assistance) is provided<br />
by <strong>FMC</strong> only as a courtesy and is intended to<br />
be general in nature. Any uses suggested by<br />
<strong>FMC</strong> are presented only to assist our customers<br />
in exploring possible applications. <strong>FMC</strong> makes<br />
no warranty, express or implied, as to its<br />
accuracy or completeness, or the results to<br />
be obtained from such information, advice or<br />
assistance. Each customer is solely responsible<br />
for determining whether the <strong>FMC</strong> products are<br />
suitable for such customer's intended use, and<br />
for obtaining any necessary governmental<br />
registrations and approvals for such customer's<br />
production, marketing, sale, use and/or<br />
transportation of finished goods using or<br />
incorporating the <strong>FMC</strong> products.<br />
Latin America<br />
Montevideo, Uruguay<br />
Tel/Fax: +5982 6043030<br />
Tel/Fax: +5982 6043104<br />
Middle East<br />
Amman, Jordan<br />
Tel: +962 6 4618150<br />
Fax: +962 6 4618156