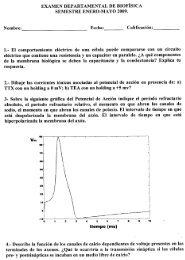
"Pteridophytes (Ferns)". In: Encyclopedia of Life Sciences
"Pteridophytes (Ferns)". In: Encyclopedia of Life Sciences
"Pteridophytes (Ferns)". In: Encyclopedia of Life Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
George Yatskievych, Missouri Botanical Garden, St Louis, MO, USA<br />
<strong>Pteridophytes</strong> (vascular cryptogams or ferns and fern allies) comprise about 12 000 species<br />
<strong>of</strong> primitive vascular plants; they do not produce flowers or seeds and reproduce instead via<br />
spores. They occur in most terrestrial habitats and also in some aquatic communities. Some<br />
species are very beneficial to humans, but the group also contains important species <strong>of</strong><br />
weeds.<br />
<strong>In</strong>troduction<br />
<strong>Pteridophytes</strong>, also known as ‘vascular cryptogams’ and<br />
‘ferns and fern allies’, comprise about 12 000 species <strong>of</strong><br />
vascular plants that do not produce flowers or seeds,<br />
reproducing instead via the production <strong>of</strong> spores. <strong>Pteridophytes</strong><br />
occur in most terrestrial habitats on earth and<br />
are also present in some aquatic communities. They are an<br />
important part <strong>of</strong> the ground vegetation in many forest<br />
communities and, with about one-third <strong>of</strong> the species<br />
growing on the trunks and branches <strong>of</strong> trees, they are also<br />
an important component <strong>of</strong> many epiphytic plant communities.<br />
Some species are very beneficial to humans, but<br />
the group also contains some <strong>of</strong> the most important weed<br />
species in the world.<br />
<strong>Life</strong> Cycle<br />
<strong>Pteridophytes</strong> are characterized by a life cycle that usually<br />
involves an alternation <strong>of</strong> two free-living generations –<br />
sporophyte and gametophyte – with the sporophyte the<br />
larger phase <strong>of</strong> the life cycle. Nonvascular plants like<br />
mosses and liverworts also have an alternation <strong>of</strong> generations,<br />
but in these organisms the gametophyte generation is<br />
generally the dominant phase. <strong>In</strong> seed plants, the<br />
gametophyte is no longer free-living but remains enclosed<br />
in tissues on the sporophyte and there is a progressive<br />
reduction in the size through various gymnosperm groups<br />
such that in flowering plants (angiosperms) the gametophyte<br />
generation is reduced to just a few cells in the<br />
germinating pollen grains and the ovules.<br />
The conspicuous phase <strong>of</strong> the pteridophyte cycle is the<br />
sporophyte, which is how most people observe the plants in<br />
nature. These are usually perennial. Sporangia are<br />
produced on the leaves <strong>of</strong> sporophytes (sometimes in<br />
specialized cone-like strobili). <strong>In</strong> true ferns, these are<br />
commonly on the leaf undersurface and are <strong>of</strong>ten clustered<br />
into discrete units called sori. Within each sporangium,<br />
specialized cells undergo a series <strong>of</strong> mitotic (structural)<br />
divisions followed by meiosis (sexual division) that results<br />
in production <strong>of</strong> spores with half as many chromosomes as<br />
in the original sporophyte. The more advanced ferns<br />
. <strong>In</strong>troduction<br />
. <strong>Life</strong> Cycle<br />
<strong>In</strong>troductory article<br />
. Reproductive Variations<br />
. Cytology<br />
Article Contents<br />
. Morphology and Anatomy<br />
. Systematics and Classification<br />
. Economic Importance<br />
usually have 64 spores per sporangium, but more primitive<br />
ferns and fern allies may have hundreds or even thousands.<br />
At maturity, the sporangium dries and ruptures, dispersing<br />
the spores into the air.<br />
When a spore lands on a suitable substrate, it<br />
germinates, the cells dividing and forming first a filament<br />
and eventually usually a heart-shaped gametophyte (sometimes<br />
other shapes in some groups) that is the same species<br />
as the sporophyte but appears very different. Gametophytes<br />
are <strong>of</strong>ten moss-like in appearance and are quite<br />
small, usually less than 1 cm wide at maturity, but are <strong>of</strong>ten<br />
fairly easily located in nature near adjacent sporophytes.<br />
Although in a few genera gametophytes can be long-lived,<br />
in most ferns their lifespan is usually much less than a year.<br />
They are the sexual phase <strong>of</strong> the life cycle in that they<br />
produce multicellular sex organs at maturity on the side<br />
away from the light. The more or less spherical antheridia<br />
(male gametangia) are produced among the rhizoids<br />
towards the base <strong>of</strong> the plant and at maturity they pop<br />
open to release motile flagellated spermatozoids. Archegonia<br />
(female gametangia) are usually produced at the<br />
opposite end near the notch region, and are flask-shaped<br />
structures containing a single egg cell. A film or droplet <strong>of</strong><br />
free-standing water is necessary in order for the spermatozoids<br />
to swim to an archegonium <strong>of</strong> the same or a<br />
different gametophyte. The neck cells <strong>of</strong> the archegonium<br />
spread at maturity and the spermatozoid swims down the<br />
archegonial canal to fuse with the egg, effecting syngamy<br />
(fertilization) and forming a zygote with twice the number<br />
<strong>of</strong> chromosomes as the gametophyte. This zygote grows<br />
and develops into a new sporophyte, completing the cycle,<br />
while the maternal gametophyte withers away.<br />
<strong>In</strong> a typical pteridophyte, each gametophyte is potentially<br />
bisexual, producing both antheridia and archegonia.<br />
Because the eggs and spermatozoids <strong>of</strong> an individual all<br />
grew from a single spore and are thus genetically identical,<br />
potentially these plants can become self-fertilized in a way<br />
that renders the resultant sporophyte entirely homozygous<br />
(having only one kind <strong>of</strong> allele for each gene locus) for its<br />
entire makeup. Various mechanisms exist to promote<br />
cross-fertilization: the gametangia <strong>of</strong>ten mature at different<br />
times; the genome may have deleterious alleles that are<br />
ENCYCLOPEDIA OF LIFE SCIENCES © 2002, John Wiley & Sons, Ltd. www.els.net<br />
1
fatal to homozygous individuals (genetic load); and there<br />
may exist mating factors that prevent successful selffertilization.<br />
Some ferns also produce pheromones known<br />
as antheridiogens, in which the first spore to germinate at a<br />
site becomes a female gametophyte and exudes a substance<br />
causing later-germinating spores to develop into male<br />
gametophytes.<br />
Reproductive Variations<br />
Unusual gametophytes<br />
<strong>In</strong> some ferns and fern allies, including some clubmosses<br />
(Lycopodiaceae), whisk ferns (Psilotaceae), and grape<br />
ferns (Ophioglossaceae), the gametophytes are not surfacedwelling<br />
and green. <strong>In</strong>stead, they are subterranean and<br />
nonphotosynthetic, <strong>of</strong>ten appearing as pale brown or<br />
yellow fuzzy cylinders or pads <strong>of</strong> tissue. These gametophytes<br />
are mycotrophic; that is, they receive their nutrients<br />
from soil-borne fungi that establish connections with their<br />
rhizoids. However, although such gametophytes are<br />
usually slow-growing, they usually produce normal<br />
gametangia and otherwise complete their life cycles in the<br />
typical fashion.<br />
Heterospory<br />
Other ferns and fern allies, including spike mosses<br />
(Selaginellaceae), quillworts (Isoetaceae), and aquatic<br />
ferns (Azollaceae, Marsileaceae, Salviniaceae), depart<br />
from the typical life cycle in producing two different types<br />
<strong>of</strong> sporangia. One <strong>of</strong> these produces numerous microscopic<br />
microspores that germinate to produce male gametophytes.<br />
The other sporangial type produces many fewer<br />
and much larger megaspores (usually visible to the naked<br />
eye), which grow into female gametophytes. <strong>In</strong> both spore<br />
types the gametophytes are reduced in structure and<br />
develop mostly within the ruptured spore wall.<br />
Vegetative reproduction<br />
Many pteridophytes supplement their sexual cycles with<br />
various forms <strong>of</strong> vegetative reproduction. This may be as<br />
simple as the fragmentation <strong>of</strong> a creeping rhizome into<br />
smaller pieces that become established as separate plants.<br />
Horsetails (Equisetaceae) growing along rivers and<br />
streams are frequently spread over long distances in this<br />
fashion by flooding. Other species develop specialized<br />
structures to effect vegetative propagation. Some ferns<br />
produce stolons, which are specialized long, spreading<br />
stems that root at their tips and form new plants. Others<br />
produce buds or bulbils on their leaves that can germinate<br />
to form new plantlets. Still others produce roots where<br />
their fronds come into contact with soil. A few species<br />
2<br />
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
produce specialized underground structures, such as tubers<br />
and similar <strong>of</strong>fsets. Some species have sporophytes or<br />
gametophytes that produce gemmae, which are specialized<br />
relatively undeveloped fragments <strong>of</strong> plants that break <strong>of</strong>f<br />
and are dispersed, eventually germinating to form new<br />
plants. <strong>In</strong> a few species <strong>of</strong> filmy ferns (Hymenophyllaceae),<br />
shoestring ferns (Vittariaceae), and other families, the<br />
ability to produce sporophytes has been lost and the plants<br />
exist only as colonies <strong>of</strong> gametophytes spreading through<br />
the production <strong>of</strong> tiny air-dispersed gemmae.<br />
Apogamy<br />
Apogamy is a widespread and important mechanism <strong>of</strong><br />
reproduction in pteridophytes more than in any other<br />
group <strong>of</strong> plants. Apogamous ferns, which frequently occur<br />
in environments with seasonal extremes <strong>of</strong> heat, cold and/<br />
or drought, avoid the necessity for sex. <strong>In</strong> the sporangia <strong>of</strong><br />
such plants, a mechanism during the series <strong>of</strong> cell divisions<br />
results in the production <strong>of</strong> spores with the same genetic<br />
constitution as the sporophyte plant (meiosis does not<br />
result in a reduction in chromosomal ploidy). These<br />
‘diplospores’ grow into gametophytes that produce new<br />
sporophytes directly from meristematic tissues near the<br />
notch region. The environmental advantages <strong>of</strong> apogamy<br />
include the faster development <strong>of</strong> the gametophyte and the<br />
release from the requirement <strong>of</strong> standing water for<br />
fertilization to take place. <strong>In</strong>terestingly, many apogamous<br />
ferns continue to produce antheridia with functional<br />
spermatozoids, which can be released and fertilize eggs<br />
on nearby gametophytes <strong>of</strong> related sexual species. Once<br />
formed, such hybrids are always apogamous and thus able<br />
to reproduce themselves.<br />
Cytology<br />
<strong>Pteridophytes</strong> characteristically have high chromosome<br />
numbers. The highest chromosome number recorded for<br />
any organism is in an adder’s tongue fern, Ophioglossum<br />
reticulatum, with about 1260 pairs <strong>of</strong> chromosomes. The<br />
base chromosome number (x) in various genera is quite<br />
variable: for example, Asplenium (x 5 36), Botrychium<br />
(x 5 45), Osmunda (x 5 22), Pellaea (x 5 29), Polystichum<br />
(x 5 41); and Pteridium (x 5 52). Exceptions occur in a few<br />
ferns, including most aquatic genera (Salvinia, x5 9).<br />
Some fern allies also have low chromosomal base numbers<br />
(Selaginella, x5 mostly 7–10).<br />
Several theories have been advanced as to why ferns have<br />
so many chromosomes. Among the most intriguing is that<br />
<strong>of</strong> palaeopolyploidy. Botanists have long known that<br />
polyploidy (the development <strong>of</strong> extra sets <strong>of</strong> chromosomes<br />
over the basic diploid level) is very widespread and<br />
common in pteridophytes. Both autopolyploidy and<br />
allopolyploidy have been documented in numerous genera.
Allopolyploidy involves hybridization between species,<br />
resulting in a sterile hybrid that regains its fertility by<br />
doubling its chromosome number during spore production<br />
in some sporangia. Autopolyploidy involves the same<br />
doubling <strong>of</strong> chromosome number during spore production<br />
but without a hybridization event. With polyploidy so<br />
pervasive among pteridophytes, geneticists have long been<br />
puzzled that most fern species are apparently functionally<br />
diploid, having only two alleles for each gene locus. <strong>In</strong> the<br />
last two decades, ‘gene-silencing’ – the selective shutting <strong>of</strong>f<br />
<strong>of</strong> duplicate copies <strong>of</strong> genes found in polyploid species –<br />
has been documented in several fern genera. This has given<br />
rise to the hypothesis that, over time, ferns undergo regular<br />
rounds <strong>of</strong> polyploidy (which increases their chromosome<br />
numbers) followed by gradual diploidization <strong>of</strong> the<br />
genomes (selective silencing <strong>of</strong> the extra gene copies to<br />
return the species to a functionally diploid level). Evidence<br />
for this mechanism is circumstantial, but it seems likely to<br />
function in at least those cases that have been better studied<br />
thus far.<br />
Morphology and Anatomy<br />
Sporophytes<br />
Stems<br />
Most ferns have specialized stems called rhizomes that are<br />
positioned at the level <strong>of</strong> the substrate or somewhat buried.<br />
Rhizomes vary greatly in size, thickness and orientation.<br />
Most commonly, they are horizontal and creeping, but<br />
many species have short upright rhizomes. <strong>In</strong> some groups,<br />
notably the tree ferns, specialized stems are trunk-like and<br />
may be 20 m or more tall. These modified stems produce<br />
only adventitious roots and are usually covered with dense<br />
scales or hairs, at least towards the growing tip.<br />
Other types <strong>of</strong> stems occur in some primitive ferns and in<br />
most fern allies. Grape ferns (Ophioglossaceae) usually<br />
have somewhat tuberous stems. Horsetails (Equisetaceae)<br />
have both rhizomes and fluted or ridged aerial stems.<br />
Quillworts (Isoetaceae) have very short stout stems with<br />
the nodes very close together (corms). Most clubmosses<br />
(Lycopodiaceae) have relatively unspecialized stems<br />
Rhizomes are structurally simpler than those <strong>of</strong> most<br />
seed plants in that they do not produce secondary growth<br />
(wood). Even the tree ferns have only primary growth, and<br />
a thick mantle <strong>of</strong> interwoven roots is produced to help with<br />
structural support. <strong>In</strong> most ferns, the vascular system <strong>of</strong> the<br />
stem is in the form <strong>of</strong> a hollow cylinder interrupted (with<br />
gaps) where traces branch <strong>of</strong>f to the leaves. <strong>In</strong> crosssection,<br />
most fern rhizomes thus appear as an irregular ring<br />
<strong>of</strong> vascular bundles. <strong>In</strong> some primitive ferns and fern allies,<br />
the vascular system is a solid uninterrupted cylinder.<br />
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
Leaves<br />
<strong>Pteridophytes</strong> exhibit an amazing variety <strong>of</strong> leaf morphologies.<br />
<strong>In</strong> most fern allies and a few primitive ferns, the<br />
leaves are reduced and scale-like, needle-like or grass-like,<br />
with at most a single vein. <strong>In</strong> most true ferns, however, the<br />
leaves are the dominant organ <strong>of</strong> the sporophyte and can be<br />
extremely complex in their pattern <strong>of</strong> division. <strong>In</strong> a few<br />
genera (especially in the Gleicheniaceae), the leaves are<br />
indeterminate in growth; that is, they continue to elongate<br />
at the tip, <strong>of</strong>ten reaching several metres in length and<br />
clambering over surrounding vegetation. The petiole<br />
(stipe) <strong>of</strong> fern leaves may be circular, angled, or U-shaped<br />
in cross-section, and is sometimes hairy or scaly. There are<br />
one to several vascular strands, and the number and<br />
position <strong>of</strong> these in the petiole are <strong>of</strong>ten diagnostic for<br />
individual families or genera.<br />
<strong>In</strong> most ferns, the development <strong>of</strong> the leaf follows a<br />
pattern known as ‘circinate vernation’. This produces a<br />
characteristic fiddlehead or crozier as the leaf uncurls. <strong>In</strong> a<br />
few genera, this pattern has become modified so that the<br />
unfurling leaf produces a hook-like structure. The fern<br />
allies and the grape ferns (Ophioglossaceae) do not exhibit<br />
circinate vernation but expand by unfolding or in an<br />
indefinite pattern.<br />
The leaf blade (lamina) varies from entire to highly<br />
divided, with pinnate, pedate and palmate patterns <strong>of</strong><br />
division in various species, but most commonly is one or<br />
more times pinnately compound. The continuation <strong>of</strong> the<br />
petiole as the central axis <strong>of</strong> the leaf blade is known as the<br />
rachis, to which the pinnae (primary divisions or leaflets)<br />
are attached. The pinnae may themselves be entire or one<br />
or more times compound. The ultimate divisions <strong>of</strong> the leaf<br />
are called pinnules, which may be entire or lobed. Venation<br />
<strong>of</strong> the leaves can be quite complex, with several orders <strong>of</strong><br />
successively finer midveins (costae) and lateral veins. The<br />
venation <strong>of</strong> the pinnules may be unbranched or branched<br />
with free to variously anastomosing veinlets.<br />
Fern leaves may be glabrous or variously covered with<br />
hairs and/or scales. <strong>In</strong> some species, the leaves are<br />
glandular and sticky. Other species secrete a powdery<br />
farina, usually on the leaf undersurface, which may be<br />
white or bright yellow or orange. The leaves also vary<br />
greatly in thickness and texture. The thinnest leaves occur<br />
in the filmy ferns (Hymenophyllaceae), in which the leaves<br />
are <strong>of</strong>ten only two cell layers thick. The production <strong>of</strong><br />
thick, leathery leaves or leaves with dense vestiture <strong>of</strong> hairs,<br />
scales, glands or farina is generally explained as adaptation<br />
to droughty habitats and/or areas <strong>of</strong> high sunlight.<br />
Sporangia<br />
<strong>In</strong> fern allies and a few primitive ferns, relatively large<br />
sporangia are produced either in complex cone-like strobili<br />
at the stem or branch tips or in the axils at the bases <strong>of</strong><br />
leaves. Some fern species produce dimorphic leaves, with<br />
vegetative (trophophyll) and fertile (sporophyll) leaves<br />
3
having different morphologies. <strong>In</strong> other ferns, the leaf is<br />
divided into specialized fertile and vegetative regions.<br />
However, in most ferns, the sporangia are produced on the<br />
undersurface <strong>of</strong> normal leaves.<br />
The positional patterns and other details <strong>of</strong> sporangia on<br />
the leaf undersurface are <strong>of</strong>ten diagnostic for particular<br />
families or genera and are a principal tool in fern<br />
classification. At one extreme, the sporangia may entirely<br />
cover the leaf undersurface (acrostichoid). <strong>In</strong> contrast, in<br />
some primitive ferns, the sporangia are sparsely scattered<br />
along some veins. <strong>In</strong> most ferns, however, the sporangia<br />
are grouped into discrete lines or clusters known as sori.<br />
Sori may be circular to linear, positioned along the margin<br />
or towards the midvein (costa), surficial or in a groove or<br />
channel, etc. <strong>In</strong> some cases, the developing sori are<br />
protected by a recurved leaf margin (false indusium), a<br />
covering <strong>of</strong> deciduous scales, or a more permanent small<br />
flap <strong>of</strong> tissue, the indusium. <strong>In</strong>dusia vary greatly in shape,<br />
size, texture and persistence, ranging from umbrellashaped<br />
to globose to linear. <strong>In</strong> the water ferns (Azollaceae,<br />
Marsileaceae, Salviniaceae), the sporangia become enclosed<br />
in hardened capsular structures called sporocarps<br />
that are formed either from modified leaflets or from<br />
modified indusia.<br />
The sporangia themselves are usually positioned on a<br />
somewhat thickened vein ending or along a portion <strong>of</strong> a<br />
vein. <strong>In</strong> most cases, the sporangium consists <strong>of</strong> a stalk, <strong>of</strong><br />
varying length and cell number, and a multicellular<br />
capsule. <strong>In</strong> most ferns, the capsule is differentiated into<br />
thin-walled cells and an annulus, a ring or region <strong>of</strong> cells<br />
with only some <strong>of</strong> the walls thickened. The annulus<br />
functions in spore release.<br />
Spores<br />
Spores are the main structures by which ferns are dispersed<br />
to form new populations. As such, in most ferns, they are<br />
relatively impervious, long-lived and metabolically inactive.<br />
Although the majority <strong>of</strong> spores produced fall within a<br />
few metres <strong>of</strong> the parental sporophyte, the spores <strong>of</strong> some<br />
ferns have been recovered from air currents in the<br />
stratosphere during high-elevation atmospheric sampling<br />
studies and ferns are among the most successful colonists<br />
<strong>of</strong> highly isolated oceanic islands. Although the life <strong>of</strong> most<br />
spores is measured in terms <strong>of</strong> months or a few years, in<br />
some cases fern spores have been induced to germinate<br />
after more than a hundred years <strong>of</strong> storage. <strong>In</strong> a few groups<br />
scattered throughout the ferns and fern allies, the spores<br />
are relatively thin-walled, green and photosynthetically<br />
active, and relatively short-lived, reflecting an adaptation<br />
to rapid establishment <strong>of</strong> new plants following dispersal.<br />
Developmentally, spores are the direct products <strong>of</strong><br />
meiosis, which begins with a single spore mother cell<br />
undergoing two separate rounds <strong>of</strong> division, and yields a<br />
tetrad <strong>of</strong> products that breaks apart into four individual<br />
4<br />
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
spores. The spatial relationship between the plane <strong>of</strong><br />
division <strong>of</strong> the two rounds <strong>of</strong> meiotic division affects the<br />
shape and markings <strong>of</strong> the resulting spores. Two main<br />
types are recognized. Trilete (tetrahedral) spores vary from<br />
nearly spherical to somewhat three-angled and have a<br />
three-branched scar where each spore was attached to the<br />
others in the tetrad. Monolete (bilateral) spores are<br />
ellipsoid to bean-shaped and have a linear attachment<br />
scar along one side. The attachment scar is usually where<br />
the spore ruptures during germination. As it matures, each<br />
spore develops two or three outer protective layers, the<br />
relatively thin endospore, the thick perispore, and in some<br />
cases an outermost exospore. The exospore and also<br />
portions <strong>of</strong> the perispore actually develop from materials<br />
produced by the inner sporangium wall and deposited over<br />
the spore, rather than by the spore itself.<br />
Mature spores vary greatly in size and surface sculpturing.<br />
Spores <strong>of</strong> some Marattiaceae are only about 15 mmin<br />
diameter, whereas the megaspores <strong>of</strong> some Selaginella<br />
species may approach 1 mm. The surface sculpturing is<br />
<strong>of</strong>ten diagnostic for various species, genera and/or<br />
families, ranging from smooth to wrinkled, spiny and/or<br />
with wing-like ridges.<br />
Gametophytes<br />
Upon germination <strong>of</strong> spores, cell divisions produce first a<br />
filamentous structure. <strong>In</strong> most ferns, this subsequently<br />
continues to divide in two or more planes and eventually<br />
differentiates into the mature gametophyte. The typical<br />
fern gametophyte is a flat, heart-shaped structure, with two<br />
lobes and an intervening notch at one end and the other end<br />
narrowed or rounded. It can vary in size from a few<br />
millimetres to about 1 cm in size. A number <strong>of</strong> variations<br />
exist but are not widespread, including filamentous, strapshaped<br />
and irregularly lobed gametophytes. <strong>In</strong> pteridophytes<br />
with subterranean mycorrhizal gametophytes, these<br />
can mature to various shapes, but most are either tubular<br />
or cushion-shaped.<br />
Gametophytes are moss-like in that they lack vascular<br />
tissue and roots. Slender hair-like structures called rhizoids<br />
function to absorb water and nutrients and act to anchor<br />
the gametophyte to the substrate. The gametangia (sex<br />
organs) generally are formed on the side <strong>of</strong> the gametophyte<br />
away from the light (except in subterranean<br />
gametophytes). The antheridia are positioned among the<br />
rhizoids and are more or less spherical structures consisting<br />
<strong>of</strong> a jacket <strong>of</strong> cells enclosing the spermatozoids. The<br />
archegonia are usually positioned near the notch on a<br />
slightly thickened pad <strong>of</strong> tissue. They are flask-shaped and<br />
somewhat sunken into the tissue. The neck <strong>of</strong> the<br />
archegonium consists <strong>of</strong> four columns <strong>of</strong> cells that separate<br />
at maturity, opening a canal and exposing the egg cell in the<br />
base for fertilization by the spermatozoid.
Systematics and Classification<br />
Classification <strong>of</strong> pteridophytes remains somewhat controversial.<br />
The terms ‘pteridophytes’, ‘ferns and fern allies’<br />
and ‘vascular cryptogams’ continue to be used informally<br />
by botanists who wish to avoid becoming enmeshed in the<br />
technical details <strong>of</strong> competing systems <strong>of</strong> fern classification.<br />
Although many <strong>of</strong> the groups <strong>of</strong> ferns and fern allies<br />
are distinctive and have been recognized since antiquity,<br />
the relationships among these groups and the taxonomic<br />
level at which they should be recognized still has not been<br />
fully resolved. <strong>In</strong> recent years, a consensus has begun to<br />
emerge, and molecular phylogenetic studies involving<br />
mostly the comparison <strong>of</strong> various gene sequences have<br />
helped to refine theories <strong>of</strong> pteridophyte evolution and<br />
taxonomy.<br />
<strong>In</strong> general, pteridophytes have a long fossil record and<br />
the main lineages trace their origins to the first vascular<br />
land plants. <strong>Pteridophytes</strong> were dominant plants in the<br />
swamps <strong>of</strong> the Carboniferous Period more than 300 million<br />
years ago, which gave rise to the world’s major coal<br />
deposits. <strong>In</strong> some groups, such as the horsetails (Equisetaceae),<br />
the relatively few modern species are the remnants<br />
<strong>of</strong> formerly much more diverse lineages. On the other hand,<br />
some modern fern species have existed for long times, as<br />
shown by the fossils indistinguishable from the modern<br />
sensitive fern (Onoclea sensibilis) dating back to the<br />
Palaeocene Epoch more than 60 million years ago.<br />
<strong>In</strong> recent years, a fundamental shift in our understanding<br />
<strong>of</strong> primitive pteridophyte classification has<br />
occurred. Traditionally, three or four main groups had<br />
been recognized. These included the clubmosses and<br />
related groups (lycophytes), the horsetails (sphenophytes,<br />
sometimes called arthrophytes), the true ferns (filicaleans),<br />
and sometimes the whisk ferns (psilophytes). The psilophytes,<br />
an unusual group with structurally relatively<br />
simple plants, were considered the most primitive group<br />
<strong>of</strong> extant vascular plants by some botanists and true ferns<br />
with reduced simplified structure by others. Recent<br />
anatomical and molecular studies have shown that the<br />
latter interpretation is probably correct – the Psilotaceae<br />
are primitive ferns whose stems, leaves and sporangia have<br />
become simplified over time. These same studies have<br />
yielded an even more fundamental conclusion. The<br />
lycophytes are apparently the most primitive group <strong>of</strong><br />
extant vascular plants. The lineage leading to the seed<br />
plants (gymnosperms and angiosperms) has its origins<br />
within the pteridophyte lineage before the divergence <strong>of</strong><br />
both the true ferns and the horsetails. Thus, the most recent<br />
hypothesis <strong>of</strong> pteridophyte evolution advocates the existence<br />
<strong>of</strong> two fundamental groups, the lycophytes and the<br />
remaining ferns and fern allies.<br />
There are a number <strong>of</strong> relatively primitive fern families,<br />
many <strong>of</strong> which are represented by relatively few modern<br />
species but some <strong>of</strong> which have extremely long fossil<br />
records. The greatest diversity <strong>of</strong> modern species exists<br />
among the most advanced fern groups, and the number <strong>of</strong><br />
families to be accepted and the relationships among these<br />
families are the topic <strong>of</strong> intensive systematic research at<br />
present.<br />
Table 1 summarizes current hypotheses concerning<br />
pteridophyte classification, from most primitive to most<br />
advanced. An estimate <strong>of</strong> the number <strong>of</strong> extant genera and<br />
species, in parentheses, follows each family name, and also<br />
the common names <strong>of</strong> selected well-known examples.<br />
Economic Importance<br />
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
Relatively few species <strong>of</strong> pteridophytes are economically<br />
important. Perhaps the best-known current use is horticultural,<br />
as garden plants, house plants and specimen<br />
plants in conservatories and greenhouses. One species,<br />
Ruhmora adiantiformis is <strong>of</strong>ten called florist’s fern; its finely<br />
divided but thick and leathery leaves resist wilting and are<br />
used in cut flower arrangements. Another horticultural<br />
practice has been the use <strong>of</strong> chunks <strong>of</strong> the dense rotresistant<br />
root mantles covering the stems <strong>of</strong> tree ferns<br />
(known as orchid bark) as a substrate for growing orchids<br />
and other plants that are epiphytic in nature. However, this<br />
has caused the decline and endangerment <strong>of</strong> numerous tree<br />
fern species, with the result that commercial trade in tree<br />
fern products is now strictly regulated by international law<br />
and trade treaties.<br />
A number <strong>of</strong> ferns have been used in handicrafts.<br />
Petioles <strong>of</strong> some members <strong>of</strong> the climbing fern family,<br />
Schizaeaceae, as well as other groups, are used in some<br />
tropical countries for colour designs in basketry and<br />
bracelets. Pteridium (bracken) leaves have been used to<br />
make a green dye. The rhizomes <strong>of</strong> the tree fern Cibotium,<br />
which are covered with dense, long, golden hairs, have been<br />
fashioned since antiquity into animal statue curios sometimes<br />
known as ‘vegetable lamb <strong>of</strong> Tartary’.<br />
One group <strong>of</strong> pteridophytes with an extensive history <strong>of</strong><br />
use is the clubmosses (Lycopodiaceae). The microscopic<br />
spores <strong>of</strong> these fern allies contain nonvolatile oils that<br />
made them useful as dry industrial lubricants. They have<br />
also been used to keep latex items such as surgical gloves<br />
and condoms from sticking together, but this practice has<br />
been mostly discontinued since it was discovered that the<br />
spores caused skin irritation and allergic reactions in some<br />
people. Other uses <strong>of</strong> the spores have been in flash powder<br />
for photography and in fingerprint powder used in forensic<br />
investigation.<br />
Various ferns are also eaten as food, with the young<br />
foliage usually steamed as a vegetable or dried and used as<br />
an additive in stews and sauces. Several species are eaten,<br />
including Diplazium esculentum (which is cultivated for this<br />
purpose in parts <strong>of</strong> Asia), but the commercially most<br />
important species in the western hemisphere is Matteuccia<br />
struthiopteris, the ostrich fern, whose fiddleheads are a<br />
5
Table 1 Summary <strong>of</strong> the classification <strong>of</strong> extant pteridophyte families<br />
Lycophytes (Division Lycopodiophyta) (4 extinct orders 1 3 extant orders (each with 1 family))<br />
Lycopodiaceae (4/380), clubmosses<br />
Isoetaceae (1/130), quillworts<br />
Selaginellaceae (1/800), spikemosses<br />
<strong>Ferns</strong> (Division Pteridophyta)<br />
Eusporangiate ferns (3 extant orders, each with 1 family)<br />
Psilotaceae (2/12), whisk ferns<br />
Ophioglossaceae (3–8/80), grape ferns<br />
Marattiaceae (4/100), giant ferns<br />
Sphenophytes (3 extinct orders 1 Equisetales)<br />
Equisetaceae (1/15), horsetails<br />
Leptosporangiate ferns (1 extinct order 1 about 10 extant orders)<br />
Primitive isolated groups (6 orders (each with 1 modern family except that Cheiropleuriaceae is in Dipteridales))<br />
Osmundaceae (3/20), cinnamon and royal ferns<br />
Hymenophyllaceae (2 or 3/650), filmy ferns<br />
Stromatopteridaceae (1/1)<br />
Gleicheniaceae (4/150), scrambling ferns<br />
Cheiropleuriaceae (1/1)<br />
Dipteridaceae (1/8)<br />
Schizaeaceae (5/200), climbing ferns, curly grass fern<br />
Heterosporous aquatic groups (Order Marsileales, also called Hydropteridales)<br />
Marsileaceae (3/70), water clovers<br />
Azollaceae (1/6), mosquito ferns<br />
Salviniaceae (1/11), water spangles<br />
Tree ferns (Order Cyatheales)<br />
Loxomataceae (2/2)<br />
Plagiogyriaceae (1/11)<br />
Matoniaceae (2/4)<br />
Metaxyaceae (1/2)<br />
Dicksoniaceae (5/28–33), tree ferns<br />
Lophosoriaceae (1/1)<br />
Hymenophyllopsidaceae (1/8)<br />
Cyatheaceae (14–20/620–675), tree ferns<br />
Advanced groups (Order Polypodiales)<br />
Lindsaeaceae (5/200)<br />
Dennstaedtiaceae (14/350), cup ferns, hay-scented fern, bracken<br />
Pteridaceae (35–45/1150), cliff brakes, lip ferns, goldback ferns, maidenhair ferns, shoestring ferns<br />
Aspleniaceae (1/800), spleenworts<br />
Thelypteridaceae (1–35/900)<br />
Blechnaceae (8/250), chain ferns<br />
Dryopteridaceae (including Tectariaceae) (30/950), wood ferns, shield ferns, halberd ferns, ostrich fern, sensitive fern<br />
Woodsiaceae (including Athyriaceae) (10–13/500), fragile ferns, lady ferns, oak ferns<br />
Lomariopsidaceae (6/550), paddle ferns, tongue ferns<br />
Davalliaceae (14/120), rabbit’s foot ferns, Boston fern<br />
Polypodiaceae (including Grammitidaceae) (35–45/1050), polypodies, staghorn ferns<br />
common sight in markets <strong>of</strong> the northeastern United States<br />
in late spring. Formerly, Pteridium aquilinum (bracken)<br />
was quite important in some cuisines, particularly in parts<br />
<strong>of</strong> eastern Asia. However, medical studies have linked this<br />
species to stomach cancer and its use has declined.<br />
6<br />
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
Perhaps the most economically valuable species <strong>of</strong><br />
pteridophyte is Azolla, a genus <strong>of</strong> tiny floating aquatic<br />
ferns. For centuries farmers in parts <strong>of</strong> eastern Asia<br />
jealously guarded strains <strong>of</strong> this plant, which they used to<br />
inoculate rice paddies in the spring for markedly increased<br />
yields. During the Vietnam War era, this practice came to
the attention <strong>of</strong> western scientists. They ‘discovered’ that<br />
hollow chambers in Azolla leaves contain symbiotic<br />
cyanobacteria (Anabaena azollae) that are able to convert<br />
atmospheric nitrogen into the nitrate form that serves as a<br />
major plant nutrient. Thus, the fast-growing plants <strong>of</strong><br />
Azolla acted as a living source <strong>of</strong> fertilizers. During the past<br />
few decades, millions <strong>of</strong> dollars have been spent to locate<br />
superior strains <strong>of</strong> this fern and to make the process more<br />
efficient, in an attempt to increase rice production in<br />
developing countries.<br />
A few ferns have had negative economic impacts because<br />
<strong>of</strong> their weediness. Two <strong>of</strong> the best examples include<br />
Salvinia and Pteridium. Salvinia molesta (Kariba weed,<br />
giant salvinia) is a floating aquatic fern that is weedy<br />
throughout the warmer parts <strong>of</strong> the world. <strong>In</strong> some<br />
situations it can form a mat several inches thick on the<br />
surface, that prevents light and oxygen penetrating into the<br />
water. <strong>In</strong> places such as New Guinea, this fern has at times<br />
threatened to destroy local fishing economies, and it is<br />
being carefully eradicated where found outside <strong>of</strong> its<br />
natural range in southern Brazil. Pteridium aquilinum<br />
(bracken) is a coarse fern with an immense creeping<br />
rhizome capable <strong>of</strong> reaching lengths <strong>of</strong> 400 m. The plant<br />
quickly invades open habitats, competing vigorously with<br />
other plants. Because the plants are toxic to livestock,<br />
bracken has ruined the pasturage on large acreages <strong>of</strong> land,<br />
especially in parts <strong>of</strong> Europe.<br />
Further Reading<br />
<strong>Pteridophytes</strong> (<strong>Ferns</strong>)<br />
Camus JM, Gibby M and Johns RJ (eds) (1996) Pteridology in<br />
Perspective. Kew, UK: Royal Botanic Gardens.<br />
Galston AW (1975) The water fern–rice connection. Natural History<br />
84(12): 10–11.<br />
Hoshizaki BJ and Moran RC (2001) Fern Grower’s Manual, revised edn.<br />
Portland, OR: Timber Press.<br />
Kramer KU and Green PS (eds) (1990) <strong>Pteridophytes</strong> and Gymnosperms;<br />
vol. 1 in Kubitzki K (ed.) The Families and Genera <strong>of</strong> Vascular Plants.<br />
Berlin: Springer-Verlag.<br />
May LW (1979) The economic uses and associated folklore <strong>of</strong> ferns and<br />
fern allies. Botanical Review (Lancaster) 44: 491–528.<br />
Perring FH and Gardiner BG (eds) (1976) The biology <strong>of</strong> bracken.<br />
Botanical Journal <strong>of</strong> the Linnaean Society 73: 1–302.<br />
Pryer KM, Schneider H, Smith AR et al. (2001) Horsetails and ferns are a<br />
monophyletic group and the closest living relatives to seed plants.<br />
Nature 409: 618–622.<br />
Sheffield E, Wolf PG and Haufler CH (1989) How big is a bracken plant?<br />
Weed Research 29: 455–460.<br />
Tryon RM and Tryon AF (1982) Fern and Allied Plants, with Special<br />
Reference to Tropical America. New York: Springer-Verlag.<br />
Wolf PG (ed.) (1995) Use <strong>of</strong> molecular data in evolutionary studies <strong>of</strong><br />
pteridophytes. American Fern Journal 85: 101–428.<br />
Wolf PG, Sipes SD, White MR et al. (1999) Phylogenetic relationships <strong>of</strong><br />
the enigmatic fern families Hymenophyllopsidaceae and Lophosoriaceae:<br />
evidence from rbcL nucleotide sequences. Plant Systematics<br />
and Evolution 219: 263–270.<br />
7