a study of alka-seltzer in different types of liquids abstract introduction
a study of alka-seltzer in different types of liquids abstract introduction
a study of alka-seltzer in different types of liquids abstract introduction
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Temperature <strong>of</strong> water (c)<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
0 20 40 60 80 100<br />
Time taken to dissolve (sec)<br />
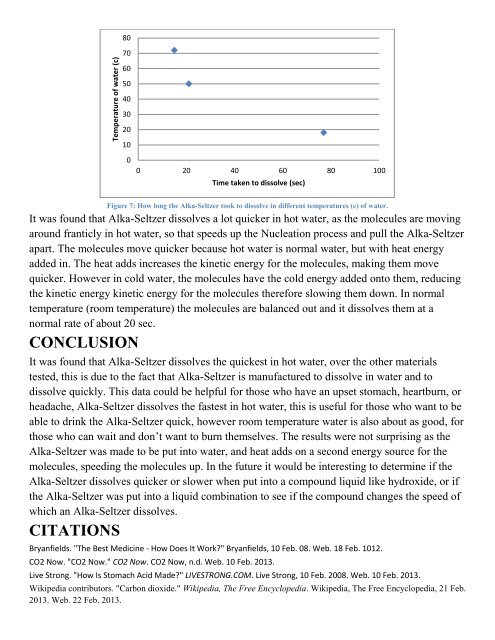
Figure 7: How long the Alka-Seltzer took to dissolve <strong>in</strong> <strong>different</strong> temperatures (c) <strong>of</strong> water.<br />
It was found that Alka-Seltzer dissolves a lot quicker <strong>in</strong> hot water, as the molecules are mov<strong>in</strong>g<br />
around franticly <strong>in</strong> hot water, so that speeds up the Nucleation process and pull the Alka-Seltzer<br />
apart. The molecules move quicker because hot water is normal water, but with heat energy<br />
added <strong>in</strong>. The heat adds <strong>in</strong>creases the k<strong>in</strong>etic energy for the molecules, mak<strong>in</strong>g them move<br />
quicker. However <strong>in</strong> cold water, the molecules have the cold energy added onto them, reduc<strong>in</strong>g<br />
the k<strong>in</strong>etic energy k<strong>in</strong>etic energy for the molecules therefore slow<strong>in</strong>g them down. In normal<br />
temperature (room temperature) the molecules are balanced out and it dissolves them at a<br />
normal rate <strong>of</strong> about 20 sec.<br />
CONCLUSION<br />
It was found that Alka-Seltzer dissolves the quickest <strong>in</strong> hot water, over the other materials<br />
tested, this is due to the fact that Alka-Seltzer is manufactured to dissolve <strong>in</strong> water and to<br />
dissolve quickly. This data could be helpful for those who have an upset stomach, heartburn, or<br />
headache, Alka-Seltzer dissolves the fastest <strong>in</strong> hot water, this is useful for those who want to be<br />
able to dr<strong>in</strong>k the Alka-Seltzer quick, however room temperature water is also about as good, for<br />
those who can wait and don’t want to burn themselves. The results were not surpris<strong>in</strong>g as the<br />
Alka-Seltzer was made to be put <strong>in</strong>to water, and heat adds on a second energy source for the<br />
molecules, speed<strong>in</strong>g the molecules up. In the future it would be <strong>in</strong>terest<strong>in</strong>g to determ<strong>in</strong>e if the<br />
Alka-Seltzer dissolves quicker or slower when put <strong>in</strong>to a compound liquid like hydroxide, or if<br />
the Alka-Seltzer was put <strong>in</strong>to a liquid comb<strong>in</strong>ation to see if the compound changes the speed <strong>of</strong><br />
which an Alka-Seltzer dissolves.<br />
CITATIONS<br />
Bryanfields. "The Best Medic<strong>in</strong>e - How Does It Work?" Bryanfields, 10 Feb. 08. Web. 18 Feb. 1012.<br />
CO2 Now. "CO2 Now." CO2 Now. CO2 Now, n.d. Web. 10 Feb. 2013.<br />
Live Strong. "How Is Stomach Acid Made?" LIVESTRONG.COM. Live Strong, 10 Feb. 2008. Web. 10 Feb. 2013.<br />
Wikipedia contributors. "Carbon dioxide." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 21 Feb.<br />
2013. Web. 22 Feb. 2013.