June 2012 - American Association for Clinical Chemistry
June 2012 - American Association for Clinical Chemistry
June 2012 - American Association for Clinical Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Towards Better Patient Care<br />
Standardization of clinical laboratory measurements<br />
improves the diagnosis and treatment<br />
of diseases. Achieving standardization<br />
requires collaboration among all groups<br />
and stakeholders involved in the measurement<br />
process and use of the measurement<br />
results. The CDC testosterone standardization<br />
program is an example of how different<br />
organizations and stakeholders can work<br />
together successfully to improve patient<br />
care and public health through better diagnosis<br />
and treatment of diseases.<br />
12 CliniCal laboratory news <strong>June</strong> <strong>2012</strong><br />
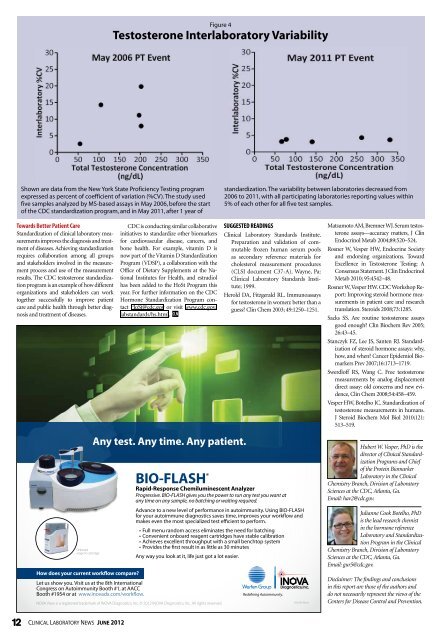
Figure 4<br />
Testosterone Interlaboratory Variability<br />
Shown are data from the New York State Proficiency Testing program<br />
expressed as percent of coefficient of variation (%CV). The study used<br />
five samples analyzed by MS-based assays in May 2006, be<strong>for</strong>e the start<br />
of the CDC standardization program, and in May 2011, after 1 year of<br />
Onboard<br />
reagent cartridge<br />
How does your current workflow compare?<br />
CDC is conducting similar collaborative<br />
initiatives to standardize other biomarkers<br />
<strong>for</strong> cardiovascular disease, cancers, and<br />
bone health. For example, vitamin D is<br />
now part of the Vitamin D Standardization<br />
Program (VDSP), a collaboration with the<br />
Office of Dietary Supplements at the National<br />
Institutes <strong>for</strong> Health, and estradiol<br />
has been added to the HoSt Program this<br />
year. For further in<strong>for</strong>mation on the CDC<br />
Hormone Standardization Program contact<br />
HoSt@cdc.gov or visit www.cdc.gov/<br />
labstandards/hs.html. CLN<br />
Any test. Any time. Any patient.<br />
standardization. The variability between laboratories decreased from<br />
2006 to 2011, with all participating laboratories reporting values within<br />
5% of each other <strong>for</strong> all five test samples.<br />
SuggeSTed ReadingS<br />
<strong>Clinical</strong> Laboratory Standards Institute.<br />
Preparation and validation of commutable<br />
frozen human serum pools<br />
as secondary reference materials <strong>for</strong><br />
cholesterol measurement procedures<br />
(CLSI document C37-A). Wayne, Pa:<br />
<strong>Clinical</strong> Laboratory Standards Institute;<br />
1999.<br />
Herold DA, Fitzgerald RL. Immunoassays<br />
<strong>for</strong> testosterone in women: better than a<br />
guess? Clin Chem 2003; 49:1250–1251.<br />
BIO-FLASH ®<br />
Rapid-Response Chemiluminescent Analyzer<br />
Progressive. BIO-FLASH gives you the power to run any test you want at<br />
any time on any sample, no batching or waiting required.<br />
Advance to a new level of per<strong>for</strong>mance in autoimmunity. Using BIO-FLASH<br />
<strong>for</strong> your autoimmune diagnostics saves time, improves your workflow and<br />
makes even the most specialized test efficient to per<strong>for</strong>m.<br />
• Full menu random access eliminates the need <strong>for</strong> batching<br />
• Convenient onboard reagent cartridges have stable calibration<br />
• Achieves excellent throughput with a small benchtop system<br />
• Provides the first result in as little as 30 minutes<br />
Any way you look at it, life just got a lot easier.<br />
Let us show you. Visit us at the 8th International<br />
Congress on Autoimmunity Booth #1, at AACC<br />
Booth #1954 or at www.inovadx.com/workflow. Redefining Autoimmunity.<br />
NOVA View is a registered trademark of INOVA Diagnostics, Inc. © <strong>2012</strong> INOVA Diagnostics, Inc. All rights reserved.<br />
690246 Rev.0<br />
Matsumoto AM, Bremner WJ. Serum testosterone<br />
assays—accuracy matters, J Clin<br />
Endocrinol Metab 2004;89:520–524.<br />
Rosner W, Vesper HW, Endocrine Society<br />
and endorsing organizations. Toward<br />
Excellence in Testosterone Testing: A<br />
Consensus Statement. J Clin Endocrinol<br />
Metab 2010; 95:4542–48.<br />
Rosner W, Vesper HW. CDC Workshop Report:<br />
Improving steroid hormone measurements<br />
in patient care and research<br />
translation. Steroids 2008;73:1285.<br />
Sacks SS. Are routine testosterone assays<br />
good enough? Clin Biochem Rev 2005;<br />
26:43–45.<br />
Stanczyk FZ, Lee JS, Santen RJ. Standardization<br />
of steroid hormone assays: why,<br />
how, and when? Cancer Epidemiol Biomarkers<br />
Prev 2007;16:1713–1719.<br />
Swerdloff RS, Wang C. Free testosterone<br />
measurements by analog displacement<br />
direct assay: old concerns and new evidence,<br />
Clin Chem 2008;54:458–459.<br />
Vesper HW, Botelho JC. Standardization of<br />
testosterone measurements in humans.<br />
J Steroid Biochem Mol Biol 2010;121:<br />
513–519.<br />
Hubert W. Vesper, PhD is the<br />
director of <strong>Clinical</strong> Standardization<br />
Programs and Chief<br />
of the Protein Biomarker<br />
Laboratory in the <strong>Clinical</strong><br />
<strong>Chemistry</strong> Branch, Division of Laboratory<br />
Sciences at the CDC, Atlanta, Ga.<br />
Email: hav2@cdc.gov.<br />
Julianne Cook Botelho, PhD<br />
is the lead research chemist<br />
in the hormone reference<br />
Laboratory and Standardization<br />
Program in the <strong>Clinical</strong><br />
<strong>Chemistry</strong> Branch, Division of Laboratory<br />
Sciences at the CDC, Atlanta, Ga.<br />
Email: gur5@cdc.gov.<br />
Disclaimer: The findings and conclusions<br />
in this report are those of the authors and<br />
do not necessarily represent the views of the<br />
Centers <strong>for</strong> Disease Control and Prevention.