Carnot cycle.pdf
Carnot cycle.pdf
Carnot cycle.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
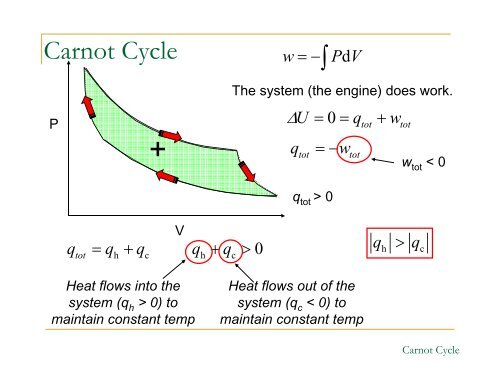
<strong>Carnot</strong> Cycle<br />
P<br />
tot<br />
h c<br />
+<br />
q = q + q qh + qc<br />
> 0<br />
V<br />
Heat flows into the<br />
system (q h > 0) to<br />
maintain constant temp<br />
w=−∫PdV The system (the engine) does work.<br />
Δ U = 0 = qtot + wtot<br />
qtot = −wtot<br />
q tot > 0<br />
Heat flows out of the<br />
system (q c < 0) to<br />
maintain constant temp<br />
w tot < 0<br />
qh > qc<br />
<strong>Carnot</strong> Cycle
w tot<br />
<strong>Carnot</strong><br />
Engine<br />
q h<br />
q c<br />
A gas that is undergoing the<br />
four stages of the <strong>Carnot</strong> <strong>cycle</strong><br />
w =− q + q<br />
tot<br />
( )<br />
h c<br />
This will be a neg. number.<br />
Which means the engine did<br />
work (that’s a good thing.)<br />
How much work is done if 50 kJ<br />
of heat flows into the engine and<br />
-40 kJ are returned to the cold<br />
source ?<br />
<strong>Carnot</strong> Cycle
Engine Efficiency<br />
Define engine efficiency as the ratio of the total work<br />
done divided by the heat input .<br />
ε =<br />
ε<br />
carn. eng.<br />
carn. eng.<br />
=<br />
w<br />
q<br />
tot<br />
h<br />
q + q<br />
q<br />
h c<br />
T<br />
ε carn. eng.<br />
= 1−<br />
T<br />
h<br />
c<br />
h<br />
q q<br />
= −<br />
T T<br />
h c<br />
h c<br />
<strong>Carnot</strong> Cycle
P<br />
<strong>Carnot</strong> Refrigerators<br />
A <strong>Carnot</strong> fridge is a <strong>Carnot</strong> Engine in reverse.<br />
w=−∫PdV The system (the fridge)<br />
requires work input. (w tot > 0)<br />
-<br />
V<br />
This makes sense, a<br />
refrigerator must remove<br />
heat from an already cold<br />
source and place it in a hot<br />
sink (the room).<br />
Heat (q h ) flows out of the<br />
system (q h < 0) to<br />
maintain constant temp .<br />
Heat (q c ) flows into the<br />
system (q c > 0) to<br />
maintain constant temp .<br />
<strong>Carnot</strong> Cycle
w tot<br />
The Room<br />
<strong>Carnot</strong><br />
Fridge<br />
q h<br />
q c<br />
The Fridge<br />
A gas that is undergoing the four<br />
stages of the <strong>Carnot</strong> <strong>cycle</strong> in reverse.<br />
q = −w<br />
tot tot<br />
But now w tot > 0, so…<br />
q < 0<br />
tot<br />
qh + qc<br />
< 0<br />
qh > qc<br />
q h < 0<br />
q c > 0<br />
More heat is dumped into the room<br />
than is removed from the fridge !<br />
<strong>Carnot</strong> Cycle
<strong>Carnot</strong> Refrigerator Efficiency<br />
η =<br />
carn. fri.<br />
η =<br />
carn. fri.<br />
η =<br />
carn. fri.<br />
q<br />
w<br />
c<br />
tot<br />
qc<br />
q + q<br />
h c<br />
Tc<br />
T −T<br />
h c<br />
q q<br />
= −<br />
T T<br />
h c<br />
h c<br />
A household refrigerator’s freezer<br />
compartment works at 255 K. Assuming that<br />
the room’s temperature is 294 K, what is the<br />
maximum efficiency ? How much work is<br />
required to remove 65 J of heat from the<br />
freezer ?<br />
<strong>Carnot</strong> Cycle