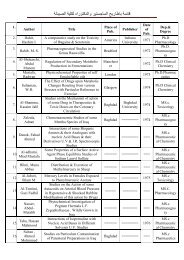
CONTENTS
CONTENTS
CONTENTS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
All right are reserved to the Iraqi Journal of Pharmaceutical Sciences<br />
<strong>CONTENTS</strong><br />
ARTICLES Page<br />
Effect of Some Storage Conditions upon the Survival of Some Fungal Spores.<br />
Raghad A.Al-Shikli , Alaa A.Abdulrasool and Mustafa M. Al-Hiti<br />
Improving an Ovulation Rate in Women with Polycystic Ovary Syndrome by<br />
Using Silymarin .<br />
Mohammed A.Taher , Yaser A.Atia and Manal K.Amin<br />
A Comparative Biochemical Study of Proteins Profile in Iraqi Children and<br />
Adolescent with β–Thalassemia.<br />
Ali M. Malik ,Emad M. Malik , Nawal MJ Al-Shammaa and Zeinab M. Al-Rubaei<br />
Comparative Effects of Fentanyl, Medazolam, Lignocaine and Propranolol on<br />
Controlling the Hemodynamic Pressor Response during Laryngoscopy and<br />
Intubation.<br />
May S. Al-Sabbagh<br />
Validity of Generalized Standard Addition Method for a Mixture of Amino<br />
Acid Analysis .<br />
Azhar M. Jasim<br />
Lithotripsy of Different Urinary Tract Stones by Using Seeds of Carum<br />
copticum.<br />
Ahmed G. Sabar<br />
Evaluation of Stability of Cefamandol and Ceftazidime with Clavulanic Acid<br />
Against Extended Spectrum β- Lactamase.<br />
Siham S. Shaokat and Hamoudi A. Hameed<br />
Gravimetric Estimation of Caffeine in Different Commercial Kinds of Tea<br />
Found in the Iraqi Market.<br />
Maha N. Hamad and Dhuha A. Abdul-Hussain<br />
Goserelin versus Norethisterone in the Management of Menorrhagia with<br />
Uterine Fibroid.<br />
Faris A. Rasheed , Jwan N. Sulaiman and Yousif Abdul-Raheem<br />
Anti-bacterial Properties of Melatonin against Mycobacterium Tuberculosis in<br />
vitro.<br />
Thamer M. Jasim , Mustafa G.Alabbassi , Suhad F. Hatem Almuqdadi and<br />
Jinan K. Kamel<br />
In vitro Evaluation of Tinidazole Bioadhesive Vaginal Gels.<br />
Zainab T.Salih<br />
Urine Protein SDS-PAGE Reveals Different Profiles in Iraqi Children with<br />
Kala-azar.<br />
Yassir MK Al-Mulla Hummadi<br />
Development of Modified Release Nicotine Tablet Formulation for Treatment<br />
of Ulcerative Colitis.<br />
Marwan Y. Al-hurr<br />
1<br />
11<br />
19<br />
24<br />
31<br />
38<br />
42<br />
48<br />
54<br />
59<br />
64<br />
70<br />
75
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
Effect of Some Storage Conditions upon the Survival of<br />
Some Fungal Spores<br />
Raghad A. Al-Shikli *,1 , Alaa A.Abdulrasool ** and Mustafa M. Al-Hiti *<br />
* Department of Clinical Laboratory Science, College of Pharmacy, University of Baghdad, Baghdad, Iraq.<br />
** Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq.<br />
Abstract<br />
Folic acid and multivitamin tablets containing Aspergillus flavus Penicillia spp. and Cladosporia<br />
spores were prepared at a compression pressure of 148 MN/m 2 and stored at 35°C under different<br />
relative humidifies (75,85, and 95)% within air tight containers, to study the effect of storage condition<br />
on the m, as well as ,the estimation of the microbial level of the raw materials intended to be used in the<br />
two kinds of tablets . Result showed that some raw materials derived from natural origin were heavily<br />
contaminated with microorganism compared to that of synthetic origin ,the results also indicated the<br />
effect of relative humidity , types of fungal spore , and the hygroscopic nature of exicpient upon<br />
survival. Multivitamin tablets showed more survival than folic acid tablets and this is due to the<br />
presence of more nutrients. No aflatoxin was obtained from both multivitamin and folic tablets at 35°C<br />
temperature; this is due to the temperature which is not an optimum temperature for aflatoxin B1<br />
production.<br />
Key words: Storage conditions of tablet, fungal spores.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﺓﺭﺍﺮﺣ ﺔﺟﺭﺩ ﻲﻓ ﺎﻬﻧﺰﺧ ﻢﺗﻭ 2ﻡ<br />
/ ﻦﺗﻮﻴﻧ ﺎﻜﻴﻣ 148 ﻂﻐﺿ ﺖﺤﺗ ﺕﺎﻨﻴﻣﺎﺘﻴﻔﻟﺍ ﻦﻣ ﺔﻋﻮﻤﺠﻣ ﺏﻮﺒﺤﻣﻭ ﻚﻴﻟﻮﻔﻟﺍ ﺾﻣﺎﺣ ﺏﻮﺒﺣ ﺮﻴﻀﺤﺗ ﻢﺗ<br />
ﺞﺋﺎﺘﻨﻟﺍ ﺖﻨﻴﺑ ﺪﻘﻟﻭ ﺏﻮﺒﺤﻟﺍ ﻦﻣ ﻦﻴﻋﻮﻧ ﺮﻴﻀﺤﺗ ﻲﻓ ﺔﻣﺪﺨﺘﺴﻤﻟﺍ ﻡﺎﺨﻟﺍ ﺩﺍﻮﻤﻠﻟ ﻲﺑﻭﺮﻜﻴﻤﻟﺍ ﺙﻮﻠﺘﻟﺍ ﺏﺎﺴﺣ ﻢﺗ ﺎﻤﻛ ﺔﻔﻠﺘﺨﻣ ﺔﻴﺒﺴﻧ ﺔﺑﻮﻃﺭﻭ ﻡ°<br />
35<br />
ﺩﺍﻮﻤﻟﺍ ﻦﻣ ﺔﺛﻮﻠﻣ ﺮﺜﻛﺃ ﺔﻴﻧﻻﺪﻴﺼﻟﺍ ﺕﺍﺮﻀﺤﺘﺴﻤﻟﺍ ﺔﻋﺎﻨﺼﻟﺍ ﻲﻓ ﻞﺧﺪﺗ ﻲﺘﻟﺍ ﺔﻴﺗﺎﺒﻨﻟﺍ ﻭﺍ ﻲﻧﺍﻮﻴﺤﻟﺍ ﻞﺻﻷﺍ ﺕﺍﺫ ( ﻡﺎﺨﻟﺍ ) ﺔﻴﻟﻭﻷﺍ ﺩﺍﻮﻤﻟﺍ ﻥﺃ<br />
ﺪﻗ ﻉﺍﻮﻧﻷﺍ ﺾﻌﺑ ﻥﺇ ﺚﺤﺒﻟﺍ ﻝﻼﺧ ﻦﻣ ﻦﻴﺒﺗ ﺪﻗﻭ .. ﺔﻴﺿﺮﻣ<br />
ﺎﻳﺮﺘﻜﺑ ﻰﻠﻋ ﺎﻬﺋﺍﻮﺘﺣﺍ ﺚﺤﺒﻟﺍ ﻦﻣ ﻦﻴﺒﺗ ﻲﺘﻟﺍ ﺍﺪﻋﺎﻣ ﻲﻋﺎﻨﺼﻟﺍ ﻞﺻﻷﺍ ﺕﺍﺫ ﺔﻴﻟﻭﻷﺍ<br />
ﻲﺘﻟﺍ ﺏﻮﺒﺤﻟﺍ ﻥﺍ ﺞﺋﺎﺘﻨﻟﺍ ﺕﺮﻬﻇﺃ ﺪﻗﻭ ﺕﺎﻳﺮﻄﻔﻟﺍ ﻩﺬﻫ ﻰﻠﻋ ﺎﻫﺮﻴﺛﺄﺗﻭ ﺔﻔﻠﺘﺨﻣ ﺕﺪﺑ ﻑﻭﺮﻇ<br />
ﺔﺳﺍﺭﺩ ﻰﻟﺍ ﺖﻋﺩ ﻚﻟﺬﻟﻭ ﺲﺒﻜﻟﺍ ﺔﻴﻠﻤﻋ ﺖﻣﻭﺎﻗ<br />
ﺔﺒﺴﻧ ﻰﻠﻋ ﺮﻬﻇﺃ ﺱﻼﺟﺭﺎﺒﺳﺍ ﺮﻄﻔﻟﺍ ﻥﺍﻭ ﺔﻴﺋﺍﺬﻏ ﺩﺍﻮﻣ ﻦﻣ ﻪﻳﻮﺤﺗ ﺎﻣ ﺐﺒﺴﺑ ﺙﻮﻠﺘﻟﺍ ﻦﻣ ﻰﻠﻋﺍ ﺔﺒﺴﻨﺑ ﺮﻬﻈﺗ ﺕﺎﻨﻴﻣﺎﺘﻴﻔﻟﺍ ﻦﻣ ﻉﻮﻤﺠﻣ ﻱﻮﺤﺗ<br />
. 1 ﺏ ﻦﻴﺴﻛﻮﺗﻼﻓﺍ ﺝﺎﺘﻧﻹ ﺔﻤﺋﻼﻣ ﺮﻴﻏ ﻡ°<br />
35 ﺔﺟﺭﺩ ﻥﺇ ﻰﻟﺇ ﺩﻮﻌﻳ ﺍﺬﻫﻭ ﻦﻴﺴﻛﻮﺗﻼﻓﻼﻟ<br />
ﺔﺒﺴﻧ ﻱﺍ ﻞﺠﺴﻳ ﻢﻟﻭ ﺕﺎﻳﺮﻄﻔﻟﺍ ﺔﻴﻘﺒﺑ ﺔﻧﺭﺎﻘﻣ ﺙﻮﻠﺘﻠﻟ<br />
Introduction<br />
The microbiological quality of nonsterile<br />
pharmaceuticals (tablets) is largely<br />
determining by the microbial contamination of<br />
a raw materials. The effect of the<br />
manufacturing process and the fate of<br />
contaminating microorganisms during storage.<br />
Several infection outbreaks which would be<br />
traced back to the use of heavily contaminated<br />
raw materials of natural origin have been<br />
reported (1) (2) (3) and (4) .During manufacturing<br />
the viability of microbial cells can be<br />
significantly affected by the drying process of<br />
granules (5) (6) (7)<br />
and by the actual compaction<br />
.The availability of water probably plays an<br />
important role. As long as tablets are stored<br />
under dry conditions spoilage due to growth of<br />
micro organisms is unlikely to occur (8) .<br />
1 Corresponding author E- mail : raghad razook@yahoo.com<br />
razook@yahoo.com<br />
Received : 7 /2 / 2009<br />
Accepted : 24 /5 / 2009<br />
1<br />
However, in regions with a hot and humid<br />
conditioned, growth of contaminating microorganisms<br />
cannot be excluded. More ever ,in<br />
such countries pharmaceutical preparations are<br />
frequently stored under uncontrolled<br />
conditions and may be dispended in non<br />
protective packaging or even without any<br />
packaging at all. Few studies that investigate<br />
the effect of storage on the microbiological<br />
quality of tablets<br />
(9) (10) (11)<br />
,but little<br />
informations are available upon the fungi as<br />
contaminants in pharmaceutical industries and<br />
possible toxicogenic power (13) (15) (16) .The aim<br />
of this study was to investigate the effect of<br />
storage under different conditions on the<br />
growth of contaminating fungal spores..
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
Materials and Methods<br />
Chemicals<br />
Acetonitrile, Acetone , Ammonium<br />
hydroxide, Anhydrous Sodium Sulfate,<br />
Benzene, chloroform , glacial Acetic acid ,<br />
hydrous disodium hydrogen phosphate<br />
(Na2HPO4 . 12H2O) . hydrochloric acid,<br />
methanol, potassium hydroxide, potassium<br />
hydroxide, potassium chloride, sulfuric acid,<br />
Sodium 1-hexana sulfonate sodium<br />
perchlorate, sodium chloride, and Tween 80<br />
(supplied by BDH England monobasic<br />
potassium phosphate from fluka-swizerland<br />
Hexana supplied by Merch-w Germany<br />
Microcrystalline cellulse (Avicel PH 101,<br />
Avicel PH 301), Folic Acid, Maize Starch,<br />
Vitamin B1 (Thiamin mononitrite), Vitamin<br />
B2 (Riboflavin). Vitamin B6 (pyridoxine<br />
HCl). Methionine, talc and Magnesium Stearte<br />
supplied by FMC corporation and Kindly<br />
supplied from (Samara Drug Industries SDl.<br />
Iraq).<br />
Microorganisms<br />
Aspergillus flavus, Penicillia SPP. And<br />
Cladsporoia Cladosporoids were obtained from<br />
College of Agriculture, University of Baghdad.<br />
Cultures were stored on Sabouraud Agar slants<br />
following incubation at 25°C for five days<br />
Fresh cultures were prepared every four weeks.<br />
Culture Media<br />
Sabouraud Dextose Agar.<br />
Rose Bengal Agar<br />
macConkey Broth<br />
solution used for dilutions and preparation of<br />
spore suspension.<br />
Relative humidity of the prepared solution.<br />
Extraction solvent of Aflatoxin.<br />
Relative humidity Containers<br />
2<br />
Relative humidity of the prepared solution.<br />
Table (1) represents the percentage of the<br />
relative humidity RH% of the prepared<br />
solutions as prescribed by AL Taher, 1990<br />
Extraction solvent of Aflatoxin<br />
According to howell and Taylor(1980) the<br />
extraction solvent of aflatoxin consists of<br />
acetonitrile : KCl 4% w/v: HCl 5N a ratio of<br />
450 ml: 10ml.<br />
Relative humidity Containers<br />
Relative humidity containers consist of two<br />
glass containers connected one above the other<br />
and joined together by the cover of them and<br />
the cover is punched to allow the exchange of<br />
humidity between the two containers .<br />
Table 1 : Relative Humidity of Various<br />
Solution<br />
Substance<br />
H2SO4<br />
KCl<br />
Na2HPO4.12H2O<br />
% of Substance<br />
in Solution<br />
23%<br />
Saturated Solution<br />
Saturated Solution<br />
Assessment of Microbial Levels of the Raw<br />
Materials and active Ingredients<br />
Sample of the following raw materials<br />
were collected from various sources and were<br />
subjected to microbiological assay according<br />
to the BP 1998 , as shown in table (2) in order<br />
to determine their microbial contents. Three<br />
types of raw materials were examined (Gelatin,<br />
Talc, and Starch) representing animal ,<br />
mineral, and botanical origin, respectively As<br />
well as those of synthetic origin such as avicel,<br />
methionine, thiamin, pyridoxine, riboflavin<br />
and folic acid .<br />
Samples were taken aseptically using the pour<br />
plate and memberane filtration techniques to<br />
determine the microbial level in the raw<br />
materials .<br />
Table 2 : Isolation and Ide tification Tests for Spe cified Microorganisms (BP , 1998)<br />
RH%<br />
Organism Enrichment Primary test Secondary test Confirmation<br />
Enterobacteriaceae Lactose broth EEB-Mossel<br />
VRBGLA<br />
Growth of Gram-<br />
35-37 °C for 2-5 hr 35-37 °C for 24-48 hr 35-37 °C for 16-24 hr negatives<br />
E.coli As above MacConkey broth MacConkey agar<br />
Indole 43.5-44.5°C<br />
43-45°C for 18-24 hr 43-45°C for 18-24 hr Biochemical<br />
Salmonella As above<br />
TBBG broth<br />
TSI agar<br />
Biochemical<br />
For 5-24 hr.<br />
42-43°C For 18-24 hr.<br />
Then subculture on:<br />
DCA,XLDA or BGA<br />
35-37 °C for 24-48 hr.<br />
35-37 °C for 18-24 hr. serological<br />
P s.aeruginosa Saline peptone Casein digest broth Cetrimide agar<br />
Oxidase test<br />
35-37 °C for 2-5 hr 35-37 °C for 24-48 hr. 35-37 °C for 24-48 hr.<br />
Staph.aureus As for P s.aeruginosa As for Ps.aeruginosa Baird-Parker<br />
Coagulase.<br />
above<br />
above<br />
35-37 °C for 24-48 hr. Catalase,DNase Test<br />
EEB-m-Mossel Enterobacteriaceae enrichment broth - Mossel ;VRVGLA,violet red bile agar<br />
75%<br />
85%<br />
95%
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
Preparation of dried spore powder<br />
A 0.1 ml aliquots of 7 days cultures of A.<br />
flavus, Cladospoa and pencillia were<br />
inoculated onto the surfaces of predried<br />
sabouraud dextrose ager plates, these were<br />
incubated at 25°C for 5 days. After this spores<br />
were clearly visible on all the plates. Three<br />
millimeters of stetrile water containing 0.1%<br />
Tween 80 (as a dispersing agent) were added<br />
to each plates. The spores were then dislodged<br />
by using glass spreader. The spore suspensions<br />
were obtained then stirred by using a vortex<br />
mixer for one minute. The spore suspensions<br />
were then filtered through a sterile cotton wool<br />
in order to get rid of the hypha. The filtrates<br />
were then harvested by centrifugation at<br />
(10,000xy) for 10 min ) the superntanat liquids<br />
were then decanted and the residues were<br />
resuspended in 20 ml of sterile distilled water,<br />
washing were repeated three times . The<br />
number of spores of the resultant spore<br />
suspensisons was determined by aviable count<br />
technique, spore suspensions were adjusted so<br />
that the following suspensions were obtained,<br />
as 2.16x106 spore/ml for A flavus, 1.68x106<br />
spore/ml forpenicillia spp. And 1.98x106<br />
spore/ml for c.cladosporoids.<br />
Tablet formulation<br />
Multivitamin tablets and folic acid tablets<br />
were prepared using the formulas listed in<br />
tables (3) and (4), respectively.<br />
The following excipients were used. Avicel PH<br />
301 as a direct compression excipient, starch<br />
(5% w/w) as a disintegrant, magnesium<br />
stearate and stearic acid as lubricants. They<br />
were mixed with the active ingredient and<br />
compressed directly using a single punch<br />
tabletting machine with 7-mm flat-faced<br />
punches<br />
Table 3 : The Formula of the Pre pared<br />
Folic Acid Tablet.<br />
Ingre dient Amount/tab.<br />
Folic Acid 1 mg<br />
Avicel PH 301<br />
118.6 mg<br />
Maize Starch 6.5 mg<br />
Mg. Stearte 2.6 mg<br />
Talc 1.3 mg<br />
Total 130 mg<br />
3<br />
Table 4: The Formula of the Prepare d<br />
Multivitamin Tablet<br />
Ingredie nt Amount / tab<br />
Tianmin Mononitrite 1.5 mg<br />
Riboflavin U.S.P. 2.0 mg<br />
Pyridoxine HCl U.S.P 2.0 mg<br />
Methionine 2.0 mg<br />
Avicel PH 301 105 mg<br />
Maize Starch 6.5 mg<br />
Taic U.S.P 6 mg<br />
Stearic Acid ( Powdered) 3.0 mg<br />
Mg.Stearte ( Powdered ) 2.0 mg<br />
Total 130 mg<br />
Preparation of contaminated tabltes:<br />
For preparation of 500 contaminated<br />
tablets (0.3 ml. 30 ml) of A. flavus (0.4 ml<br />
40ml) of penicillia spp and (0.3ml, 30ml) of C.<br />
cladosporoids were transferred to a sterile<br />
mortars and placed in the incubator until<br />
completely evaporation of water. Dried spores<br />
were scraped off and were included in direct<br />
compression formulations by dry mixing to get<br />
10 2 spore /gram and 10 4 spore/gram for each of<br />
A. flavus pencicilia spp and C. cladosporoids<br />
respectively. Ingredients including dried<br />
microorganisms spores were weighed and<br />
lightly mixed in a glass morter by the method<br />
of geometric dilution technique for 20 minutes<br />
preliminary experiments had established that<br />
this method gives a uniform distribution of the<br />
microorganisms within the formuation screen<br />
in the lubricant (magnesium stearte or stearic<br />
acid) and mixed for an additional 5 minutes .<br />
Quantities each of 130 mg were accurately<br />
weighed and poured into 7-mm diameter<br />
compresed between flat –Faced punces using a<br />
single punch tableting machine which was<br />
disinfected with 70% alcohol and the feed shoe<br />
was heat sterilized before use.<br />
Determination of viable number of spores in<br />
the prepared tablets:<br />
Viable number of spores in prepareted<br />
tablets was determined immediately after their<br />
production at different compression forces and<br />
after storage up to 8 weeks eight tablets (total<br />
wt.=1gm) were disintegrated in tryptic soy<br />
broth (9ml) according toBP 1998 using a flask<br />
shaker and suitable serial dilution in tryptic<br />
broth were prepred. One –ml sample of each<br />
dilution was poured in sterile petridish and<br />
then 15 ml of molten dextrose agar was added<br />
to the plate.
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
The sample and molten sabouraud dextrose<br />
agar were mixed together in forward and<br />
backward movement and swirled movement.<br />
The plate were allowed to solidify on surface .<br />
the plates were incubated at 35°C for 2-5 days.<br />
Survivals as colony forming units were<br />
estimated as the mean of triplicate<br />
determination and expressed as a percentage<br />
relative to an uncompressed control samples of<br />
the contaminated formulation.<br />
Physical properties of tablets:<br />
Thirty tablets were prepared using<br />
different compression pressure (137.9, 144.8<br />
and 148.3MN/m 2 ) the physical properties of<br />
the tablets were determine (plumpton, 1982),<br />
these are tablet weight, thickness, friability,<br />
hardness (breaking strength), and<br />
disintegration time<br />
Assay of Tablets:<br />
An HPLC method was used for the assay<br />
of multivitamin tablets and folic acid tablets.<br />
Assay for pyridoxine hydrochloride and<br />
thiamin multivitamin Tablets<br />
The assay for pyridoxine hydrochloride<br />
and thiamin in multivitamin tablets was done<br />
according to the U.S.P XXIV method.<br />
Effect of storage under different Relative<br />
Humidities upon the Survival of A. flavus,<br />
Penicilline, and Cladospori in folic acid and<br />
Multivitamin Tablets<br />
Folic acid and multivitamin tablets<br />
containing Aspergillus.flavus Cladosporia ,and<br />
penicillia spores prepared at a compression of<br />
148 MN/m2 and stored at 35°C under different<br />
relative humidity's (RH). The relative humidity<br />
were 75%, 85% and 95% within airtight<br />
containers. Survival of the spores within the<br />
tablets was assessed as the mean viability for<br />
each group at time 0 and after 1,2, 4,6 and 8<br />
weeks. The total viable counts of the<br />
uninoculated (control) folic acid and<br />
multivitamin tablets were measured directly<br />
after preparation and after 4,8 weeks of storage<br />
at 35°C and 75% RH, 85% RHor 95% RH.<br />
Aflatoxin assay<br />
The amount of aflatoxin produced after<br />
storage the tablets (multivitamin and folic<br />
acid) at different relative humidities (75,85 and<br />
95)% were assed at different time intervals<br />
using a modified method of Howel and Taylor<br />
(1981), the modification includes the use of<br />
twenty five grams of multivitamin and flic acid<br />
tablets stored at 4,6 and 8 weeks intervals.<br />
4<br />
Results and Discussion<br />
Microbiological quality of some raw<br />
materials used in the production of tablet and<br />
tablet ingredients<br />
Microbiological evaluation of the raw<br />
materials used in the production of tablets is<br />
presented in table (5), the result show that<br />
synthetic materials such as folic acid,<br />
magnesium stearate ,thiamin, riboflavin,<br />
pyridoxine, methionine and microcrystalline<br />
cellulose (avicel PH 301) had no microbial<br />
contaminants thus they meet the requirement<br />
of the B.P 98 which specify that atotal viable<br />
aerobic count bacteria should be equivalent to<br />
or less than 103 c.f u/gm and a total viable<br />
count for fungi equivalent to or to less than<br />
102 c.f.u/gm is accepted. Microcrystalline<br />
cellulose PH 101 although it is a syntheyic raw<br />
material , it showed the presence of<br />
pseudoumonas aeruginosa (2x 10 2 cfu/gm) .<br />
samples taken from different parts of the<br />
microcrystalline PH 101 container showed<br />
apresence of pathogens in upper part of the<br />
container this is because microcrystalline<br />
(MCC) is a highly hygroscopic material (17)<br />
through its capillary action when it is exposed<br />
to air .Table (5) also shows that raw materials<br />
of natural origin(maize starch ,gelatin and talc)<br />
had relatively higher microbial levels which<br />
are (7*10 2 ,9*10 2 and 102 C.F.U/gm)for starch,<br />
gelatin and talc, respectively than that of the<br />
synthetic origin. This finding is similar to that<br />
of lbrahim Y.K.E 1991 which is due to the<br />
fact that materials of natural origin is rich in all<br />
the necessary requirement of growth needed by<br />
the microorganism. in addition, the results<br />
indicate that raw materials derived from<br />
animal and botanical origin had a higher<br />
microbial level than that o f mineral origin.<br />
This is in agreement with the reported<br />
hypothesis of Bonomi and Negrriti,Baggerman<br />
and Kannegiter<br />
(22),(23),(3)<br />
.However, the<br />
microbial level obtained still below the B.P<br />
1998 requirements.
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
Table 5 : Microbial Contamination Levels in Some Pharmaceutical Raw Mate rials for the<br />
Production of Folic Acid and Multivitamin Table ts<br />
* C.F U/gm Starch Ge latin Talc<br />
Total Viable Aerobic Count 710 2<br />
910 2<br />
bacillus<br />
5<br />
Avicel<br />
PH PH<br />
301 101<br />
Mag<br />
Steara.<br />
Folic acid<br />
B1,B2,B3<br />
Me thionine<br />
10 2 ** ** **** ****<br />
Total Viable Count for Fungi **** **** **** ** ** **** ****<br />
Enterobacteriaces **** **** **** ** ** **** ****<br />
Escherchia coli **** **** **** ** ** **** ****<br />
Staphylucocus aureus **** **** **** ** ** **** ****<br />
Pseudomonas aerugenosa **** **** **** ** ** **** ****<br />
Salomonella **** **** **** ** ** **** ****<br />
Tablet Evaluation:<br />
Folic acid and multivitamin tablets were<br />
prepared as previously mentioned (tables 3 and<br />
4 respectively). The prepared folic acid and<br />
multivitamin tablets were evaluated physically,<br />
chemically and microbiologially. The results<br />
are shown in table 6.<br />
Table 6 : Physical Che mical and Microbiological ( Control ) Evaluation of Folic acid and<br />
Multivita min Tablet<br />
Tablet Evaluation Multivitamin Folic acid<br />
Weight<br />
(7 mm )<br />
130 mg 130 mg<br />
C. Pressure 148.3MN/m 2 148.33MN/m 2<br />
Wt. Uniformity 0.9% 0.9%<br />
Hardness (Kp) 6.6 0.4 6.5 0.6<br />
Thickness(mm) 2.85 2.85<br />
Friability % 0.2 0/2<br />
DisintegrationTime Assay 2.3 min 2 min<br />
B Pyridoxine % 133.78%<br />
B1 Thiamin % 148.2%<br />
Folic acid % 90%<br />
Microbiological Quality<br />
One day after preparati less than 10 * CFU/gm<br />
After storage for 8 weeks at 35 C , 75 % RH less than 10 CFU/gm<br />
After storage for 8 weeks at 35 C , 85 % RH less than 10 CFU/gm<br />
After storage for 8 weeks at 35 C , 95 % RH less than 10 CFU/gm<br />
Effect of relative humidity upon the survival<br />
of .A flavus in multivitamin and folic Acid<br />
tablets<br />
Figure1,2 show the effect of storage<br />
under different relative humidities (95,85 and<br />
75)% upon survival of Aflavus. spores in<br />
multivitamin and folic acid tablets. The results<br />
indicate that at a contamination level of 10 4<br />
spore/gm as shown in figure(1) and storage at<br />
75% R.H a decrease in survival over the eight<br />
weeks storage period to 13% whilest storage at<br />
95% RH, caused an initial reduction in<br />
viability followed by a substantial increase to<br />
86% at the end of 8 weeks in multivitamin<br />
tablets. Agermination, mycelia growth and<br />
sporulation occurred.The same behavior with<br />
folic tablets but with lower percentages.<br />
Visible fungal growth and sporulation were
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
apparent on the tablets after 6 weeks of<br />
storage. Further storage for 8 weeks caused a<br />
tablets to afragment. Storage at 85% R.H also<br />
showed visible fungal growth after 6 week.<br />
Viability of the organisms decreased during<br />
the first week of storage. This was<br />
accompanied by visible signs of mycelia<br />
growth. The decreased viability was probably<br />
due to the transition from dormant to mycelia<br />
state. Tablets containing hygroscopic materials<br />
are much more to physically, adsorb<br />
substantial amounts of water. Avicel has the<br />
ability to pick up moisture by capillary action<br />
and loosening of inter particulate hydrogen<br />
bonds on exposure to high humidities (8) .The<br />
water requirements for microorganisms varies<br />
depending on the organism (Blair,1988) as<br />
mentioned before. For different mould spores<br />
the minimum R.H required for germination<br />
varies from 70 to 98% and the optimum<br />
temperature for growth of moulds vary from<br />
(23-40) °C.For Aspergillus, 80% humidity<br />
aw=0.81 (23) is essential for spore germination<br />
and the optimum temperature is (30-40)<br />
°C.Multivitamin tablets show higher growth<br />
than folic acid tablets within the storage time<br />
especially 95%, R.H this may be due to<br />
presence of more nutrients like vitamins<br />
(B1.B2 and B3)carbon source (starch) and<br />
amino acids (metithionine) or what is called<br />
nutrient availability. When nutrients are<br />
abundant, growth will be sustained but when<br />
only atrace nutrients are present, growth will<br />
be minimal. Fungi need various nutrients in<br />
order to meet their energy needs and to form<br />
macromolecules such as proteins and DNA.<br />
Since fungi cannot synthesize carbohydrate, so<br />
the substrate showed contain these compounds<br />
; however, they can growth in a substrate rich<br />
in proteins without carbohydrates , e.g. cheese<br />
, by using amino acids as carbon source.<br />
Another important nutrient is nitrogen . All<br />
fungi can assimilate organic nitrogen<br />
compounds, depending on the species , certain<br />
vitamins must also be present in substrate,<br />
while the fungus itself synthesized others. The<br />
most important factors for growth are<br />
temperature , water activity and oxygen<br />
besides the presence of nutrients. Figure 2<br />
shows the effect of storage upon the percent of<br />
survival using 10 2 spore/gm of A. flavus in<br />
multivitamin and folic acid tablets.The results<br />
indicate that storge at 75% and 85%R.H<br />
showed a decrease in number of spores to zero<br />
percent in eight weeks duration whilst storage<br />
at 95% showed increase in number of spores to<br />
420 percent for the same time. The result also<br />
indicate that there is a significant different<br />
(p
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
The effect of R.H upon survival of Penicillia<br />
spp. Spores in multivitamin and folic acid<br />
Tablets:<br />
Figures 3,4 show the effect of relative<br />
humidities upon survival of penicillia spp.<br />
Spores in multivitamin and folic acid tablets.<br />
Figure 3 : Effect of relative humidity upon<br />
survival of Pe nicillia SPP. (10 4 spore / gm )<br />
compacted in multivita min (mv) tablet and<br />
folic acid (fa ) tablet at (148.3 MN/m 2 ) and<br />
store d at 35C . L.S.D0.05 = 27.87.<br />
100<br />
Figure 4 : Effect of relative humidity upon<br />
survival of Pe nicillia SPP. (10 2 spore / gm )<br />
compacted in multivita min (mv) tablet and<br />
folic acid (fa ) tablet at (148.3 MN/m 2 ) and<br />
store d at 35C . L.S.D0.05 = N.S.<br />
The results indicate that for both<br />
contamination levels of 10 4 and 10 2 spore/gm,<br />
storage at 75%RH, more decrease in survival<br />
of penicillia over the eight weeks storage<br />
period than A. flavus. was obtained i.e the<br />
decrease was to 1.8% and zero for 10 4 and 10 2<br />
spore/gram, respectively. Whilst storage at<br />
95% R.H however caused an initial reduction<br />
in viability followed by a substantial increase<br />
as germination mycelial growth and<br />
sporulation occurred for 10 4 spore/gram.<br />
Visible fungal growth and sporulation were<br />
apparent on the tablets after six weeks storage<br />
but the survival level was less than A. flavus.<br />
Further storage for eight weeks caused the<br />
tablets to fragment. On the other hand, storage<br />
at 85% R.H both contamination level 10 2 and<br />
10 4 spore/gm, no visible fungal growth was<br />
apparent on the tablets after storage for eight<br />
weeks. Penicillia spp.. 80% humidity is<br />
essential for spore germination aw=0.84 (23)<br />
and the optimal temperature is (25-30) °C for<br />
most penicillia spp. The maximal temperature<br />
is (28-35) °C .the result also show that<br />
multivitamin tablets have more survival than<br />
folic acid for the three relative humidites used.<br />
So, the over all data indicate that there is a<br />
significant difference (p
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
100<br />
Figure 5 : Effect of relative humidity upon<br />
survival of Penicillia SPP C. Clado (10 4<br />
spore / gm ) compacte d in multivita min<br />
(mv) tablet and folic acid (fa ) table t at<br />
(148.3 MN/m 2 ) and stored at 35C . L.S.D0.05<br />
= N.S.<br />
100<br />
Figure 6 : Effect of relative humidity upon<br />
survival of Penicillia SPP C. Clado (10 2<br />
spore / gm ) compacte d in multivita min<br />
(mv) tablet and folic acid (fa ) table t at<br />
(148.3 MN/m 2 ) and stored at 35C . L.S.D0.05<br />
= N.S.<br />
The obtained results are in contrast to the<br />
results obtained by Fassihi and Parker (24) ,who<br />
found that tablets stored at a lower temperature<br />
(25°C) and at 96% R.H did not spoil due to<br />
mould growth (Aspergillus niger and penicillia<br />
spp) when stored under these condition and the<br />
viability of mould spores decreased slightly .<br />
On the other hand the results are in agreement<br />
with that of the study of Bos (19) in which a<br />
visible growth of A. Niger during storage at<br />
100 and 95% R.H of sta-RX tablets and<br />
lactose/starch tablets stored at 25°C and 31°C<br />
respectively was obtained.<br />
If contaminants are introduced into tablets<br />
prior to processing (i.e. from the raw materials)<br />
then they might sitll eventually be responsible<br />
for the spoilage of the finished products . The<br />
nature of contaminating organism , the relative<br />
humidity at which tablets are stored and the<br />
tablet nutrient all contribute to survival of the<br />
organisms .<br />
Aflatoxin Assay<br />
Aflatoxin production is highly affected<br />
by type of substrate, the presence of minerals,<br />
the humidity and temperature (26 - 29) .Results<br />
were obtained from thin layer chromatography<br />
(TLC) and in camparison with standard<br />
showed that both multivitamin tablets and folic<br />
acid tablets contain no aflatoxin B1.If the<br />
environmental conditions (temperature, and<br />
relative humidity) are not suitable for fungal<br />
growth this will lead to decrease in aflatoxin<br />
production to a level that cannot be detected.<br />
Storage of tablets at 75% RH showed no<br />
aflatoxin B1 production, this result is in<br />
agreement with WHO (30) which determins that<br />
83-85% RH is an optimum RH for AFB1<br />
production by A. Flavus also this result is<br />
consistent with that obtained by Austwish (31)<br />
that determined 85% RH and more at<br />
temperature of 25°C is an optimum RH for A.<br />
flavus growth in addition, lakshinarasimham,<br />
and (32) determined that 20°C and RH 73.5%<br />
are considered as an optimum conditions for<br />
storage without fungal contamination.<br />
Furthermore storage at 85% RH showed no<br />
aflatoxin production, although RH is considerd<br />
as optimum for A. flavus growth but storage at<br />
35°C is not optimum temperature for aflatoxin<br />
production since the optimum temperature for<br />
aflatoxin is (25-28)°C (33, 34) .Also, no aflatoxin<br />
production was noticed when storage at 95%<br />
RH although RH is considered as an optimum<br />
for A. flavus growth but 35°C is not an<br />
optimum temperature for aflatoxin production .<br />
Multivitamin tablet which contanins amino<br />
acid also show no aflatoxin production this<br />
because the storage temperature is not an<br />
optimum temperature for aflatoxin production<br />
so both an optimum temperature and relative<br />
humidity required for aflatoxin production .<br />
Conclusions<br />
The results showed the existence of<br />
relationship between type of the raw materials<br />
used in pharmaceutical production and its<br />
microbial level . The results showed the effect<br />
of various storage conditions upon survival,<br />
which depends upon the type of fungal spore,<br />
the hygroscopic nature of the excipient and the<br />
relative humidity of storage. Multivitamin<br />
tablet showed more survival than folic acid<br />
tablet and this is due to the presence of more<br />
nutrient. And finally no aflatoxin was obtained<br />
for both multivitamin and folic acid tablets at<br />
35°C temperature, this is due temperature
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
which is not an optimum temperature for<br />
aflatoxin B1 production.<br />
Reference<br />
1. Kalliugetal. Kallings, L.O: ringeretz o<br />
silverstorage : and emr feldt F.<br />
Microbiologgical contamination of<br />
medical preparations, Actapharma<br />
Suec1966: 3:219-228.<br />
2. Sinugh p1 arirastaia B, kuma. A and<br />
dubey, N.K., Microb E.coli, , 2008 (56) :<br />
555-560.<br />
3. Kimiko farmati cheskii ehumnali,<br />
surivalal of microscopic fungi mnonsierile<br />
medical prrpation and auxillary subsiances<br />
, 2006: 40: 54-56<br />
4. Obuexaeco, obekwe I.F. , ogbimi AO<br />
actapol. Pharm- drag res. , 2001: 53<br />
5. Parker MT. Jowrnal of the Society of<br />
cosmetic chemists, 1978 : 23 :415<br />
6. Fassihi, A.R., and parker, M.S inimicable<br />
effects of compaction speedon micro<br />
organisms in powder systems with<br />
dis_simiilar compaction mechanisms<br />
J.phase SCI , 1987 :76: 466-470<br />
7. Plumpton EJ Gilbat,p, and fell JT the<br />
survival of micro organisms during<br />
tableting INT.J pharm, 1986a, 30 :241-<br />
146.<br />
8. Blair, T.C, buckton, g, and bloomfiled,<br />
SF baird RJ hR. heak R.f and leachr (edj)<br />
microbial quality assurance pharmacent<br />
calts cosmeticals cosmetics and tioletric<br />
eis horwood chichest ppi 1988,104-116.<br />
9. Blair , T.C graham bucton, sally F.<br />
bloomfield on the mechanism of kill of<br />
microbial of contanints during tablet<br />
compression int –jpharm ,1991:72: 111-<br />
115.<br />
10. Fassihi A.R ,and palkar M.S the influence<br />
water activity and oxygentension upon the<br />
survival of aspergilly and penisillia<br />
species and tablet INT bio detior bull B,<br />
1977:75-80<br />
11. Waterman R.f sumner EFbldwn JN and<br />
warren F.W survival of stupply lococcus<br />
aureson pharmaceutical solid dosage form<br />
J. pharmsei , 1973 ,621 1317-1320.<br />
12. Goda, n. k.s v. malath and R.uu suganthi<br />
effect of some chemical and herbal como<br />
under ongrowth of aspergillus parsiticesv<br />
and of latoxin production Animal feed<br />
science of technology , 2004,116:281-291.<br />
13. abdullaMH1988 the isolation of aflatoxin<br />
from acacia and the incidence of<br />
Aspergillus flavus in the Sudan<br />
Mycopathologia, ; 1988, 104: 143-144.<br />
14. Hitokoto, H 1978 H: muruzomi, s: Wauke,<br />
Tsakai, S: and Kuratah, Fungal<br />
9<br />
contaminand mycotoxindetection of<br />
powdered herbal drugs Appl.<br />
15. Wall heaver K,H micrological as aspects<br />
on the subject of oral solid dosage from<br />
pharm . Ind , : 1977 , 39 491-497.<br />
16. AL- HiTi 1998 M.M.A Al janabi S. the<br />
effect of some peservits on preservative on<br />
Aspergilliaus flavus growth and its<br />
aflatoxin production Irqi J.pharm Scivol<br />
(10): 1998.<br />
17. Aulton 2000 et., Me,: Tebby. H,G white<br />
p.J.p journal pharm, pharmacol, 26 suppl,<br />
59p-60p 2000 Blair, T.C Graham Bucton;<br />
Sally F,: bloomfield on the mechanism of<br />
kill of microbial contaminants during<br />
tablet compression – Int –J- pharm , 1991.<br />
72: 111- 115 .<br />
18. Alfonoso 1985<br />
19. Bos 89, C,E van doorne ,H lerk C.F. on<br />
microbiological stability of tablets stored<br />
under tropical conditions, : 1989 , 55: 175-<br />
183 .<br />
20. Ibrahim Y.K.E, 1991Y.K.E; orinolou- pdf.<br />
Comparative microboial levels in levels in<br />
wet granulation and direct compression<br />
method of tablet production, ACTA<br />
HELV. : 1991 66: 11.<br />
21. Bonomi, E. and nergetti, F. 1977 Bonomi<br />
E. and Nergetti, F. studies on the<br />
microbial content of raw materials used in<br />
pharmaceutical preparation Ann. 1 st .<br />
super Sonita: 1977, 13:802-832.<br />
22. Panlo. J Brasillianj of microbiology , 2006<br />
,vol. 37.no 1.<br />
23. Northolt MD and bull ermine prevention<br />
of moldgrothed and Bullerman prevention<br />
of moldgroth and tex production through<br />
control of environmental condition .J. too<br />
production , 1982 ,45: 519- 526.<br />
24. Fassihi and parker 1977 fassihi A.R and<br />
parker M.S the of water activity and<br />
oxygen tension upon the survival of<br />
aspergillus and penicllia tablet INT<br />
Biodeterior Bull.1977 , 13-80<br />
25. Plumpton 1982 E.J studies upon the<br />
survival of various microorganism in solid<br />
dosage froms Ph.D. thesis university of<br />
Manchester. 1982<br />
26. Reddy S.V M.D Kiram R.M. uma, K. thir<br />
umaladai and D.V.R ready aflatoxin B<br />
different grades of chillies, 2001, 18: 553-<br />
558,<br />
27. Elad. D, .risk uma, assessment grades of<br />
malicious bio contamination of food<br />
tournal food protection, 2005, 68:1302-<br />
1305.<br />
28. Shar pirar.o. control of mycotoxin storage<br />
and technigagus for their decontaminal my<br />
cotoxin in food detection and control.
Iraqi J Pharm Sci, Vol.19(2) 2010 Fungal contamination and storage conditions<br />
Wood head publishing Cambridge u.K- 2004, 190-223.<br />
29. Northolt M.D. the effect water activity and<br />
temperature the production of some my<br />
cotoxines Diss wageninger 1979<br />
30. WHO, 2003. Laboratory manual 2 nd<br />
ediction interim guidelines WHO.<br />
31. Austwish , p.k.c; and ayerst, G.groundaut<br />
micrflora and toxicily –chem. Ind 1963<br />
,55 -61.<br />
32. Mukher 95 :and lakshminarasimiham A.B<br />
aflatoxin contamination of storage under<br />
10<br />
controlled conditions int J med Microbiol<br />
Virol parassitol infect dis ., 1995 , 282(3):<br />
387-43.<br />
33. Bennet Jw, klichm my cotoxins- chinical<br />
microbiology review ; 2003,16:497-516.<br />
34. Paterson rrm; venancio A; liman solution<br />
to penicillium taxonomy crucy to my<br />
eotoxin rerearch and heath research in<br />
microbiology ; 2004 ,155: 507-513.
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
Improving an Ovulation Rate in Women with Polycystic Ovary<br />
Syndrome by Using Silymarin<br />
Mohamme d A.Taher* ,1 , Yaser A.Atia** and Manal K.Amin***<br />
*Department o f Clinical Laboratory Sciences, College of Pharmacy, University of Baghdad,Baghdad,Iraq<br />
** College of Alkindney Medicine ,University of Baghdad, Baghdad, Iraq .<br />
*** Ministry of Health, Baghdad, Iraq.<br />
Abstract<br />
Polycystic ovary syndrome(PCOS) is a heterogeneous disorder of uncertain etiology , it is the most<br />
common endocrinopathy in women and most common cause of anovulatery infertility ,characterized by<br />
chronic anovulation and hyperandrogenemia .The present study was designed to investigate the effect of<br />
silymarin which is known to have antioxidant and insulin sensitivity effects on the levels of glucose,<br />
insulin ,testosterone ,leutinizing hormone(LH) and progesterone .Ovulation rate and Homeostasis Model<br />
Assessment of insulin Resistance (HOMA) ratio were determined .A 3-months of treatment were conducted<br />
in 60 PCOS patients in three well-matched groups .The first one (n=20),received silymarin(750mg/day)<br />
.The second group received metformin(1500mg/day) while the third group treated by<br />
combination of metformin (1500mg/day )and silymarin (750mg/day). All these groups had taken the<br />
drugs in divided doses. The results showed significant improvement in all parameters at the end of treatment<br />
.The percentage of increment in progesterone levels after completion of treatment were 12.12, 15.9,<br />
and 17.51 in groups 1, 2, and 3 respectively and the number of patients ovulated after 3 months of treatment<br />
were 4,5, and 10 in groups 1,2, and 3 respectively. However they are more better in group of patients<br />
who were treated with combination of silymarin with metformin.In conclusion the addition of silymarin<br />
to metformin in treatment of PCOS patients has improving effect on disturbed hormones and<br />
ovulation rate.<br />
Key words: Polycystic ovary syndrome, silymarin, ovulation rate,metformin<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﺐﺒﺳ ﺮﺜﻛﺃﻭ ءﺎﺴﻨﻟﺍ ﺪﻨﻋ ﻊﺋﺎﺷ ﻲﻧﻮﻣﺭﻮﻫ ﻝﻼﺘﻋﺍ ﻪﻧﺍ.<br />
ﺓﺪﻛﺆﻣ ﺮﻴﻏ ﺏﺎﺒﺳﻷ ﺮﻳﺎﻐﺘﻣ ﺏﺍﺮﻄﺿﺍ ﻮﻫ ﺱﺎﻴﻛﻷﺍ ﺩﺪﻌﺘﻣ ﺾﻴﺒﻤﻟﺍ ﺔﻣﺯﻼﺘﻣ ﻥﺇ<br />
ﺮﻴﺛﺄﺗ ﺺﺤﻔﻟ ﺖﻤﻤﺻ ﺔﻴﻟﺎﺤﻟﺍ ﺔﺳﺍﺭﺪﻟﺍ ﻥﺃ.<br />
ﻱﺮﻛﺬﻟﺍ ﻥﻮﻣﺭﻮﻬﻟﺍ ﻉﺎﻔﺗﺭﺍﻭ ﻦﻣﺰﻣ ﺔﺿﺎﺑﺍ ﻡﺪﻌﺑ ﺰﻴﻤﺘﻳﻭ ﺔﺿﺎﺑﻻ ﺍ ﻡﺪﻌﺑ ﺏﻮﺤﺼﻤﻟﺍ ﻢﻘﻌﻠﻟ ﻊﺋﺎﺷ<br />
, ﻦﻴﻟﻮﺴﻧﻷﺍ , ﺮﻜﺴﻟﺍ ﺕﺎﻳﻮﺘﺴﻣ ﻰﻠﻋ ﻦﻴﻟﻮﺴﻧﻸﻟ ﺔﻣﻭﺎﻘﻤﻟﺍ ﻞﻴﻠﻘﺗﻭ ﻦﻴﻟﻮﺴﻧﻷﺍ ﺲﺴﺤﺘﻟ<br />
ﺓﺩﺎﻳﺯ ﻭ ﺪﺴﻛﺄﺘﻟﺍ ﺪﺿ ﺔﻴﺻﺎﺧ ﻚﻠﻤﻳ ﻱﺬﻟﺍ ﻮﻫﻭ ﻦﻳﺭﺎﻤﻠﻴﺴﻟﺍ<br />
(HOMA ) ﻦﻴﻟﻮﺴﻧﻸﻟ ﺔﻣﻭﺎﻘﻣ ﻞﻴﻟﺩﻭ ﺔﻇﺎﺑﻻﺍ ﺔﺒﺴﻧ ﺏﺎﺴﺘﺣﺍ ﻢﺗ . ﻥﻭﺮﻴﺴﺘﺴﻴﺟﻭﺮﺒﻟﺍﻭ ﻲﻨﻴﺗﻮﻠﻟﺍ ﻥﻮﻣﺭﻮﻬﻟﺍ , ﻱﺮﻛﺬﻟﺍ ﻥﻮﻣﺭﻮﻬﻟﺍ<br />
20 ﻦﻣ ﺔﻧﻮﻜﻣ ﻰﻟﻭﻷﺍ ﺔﻋﻮﻤﺠﻤﻟﺍ . ﻊﻴﻣﺎﺠﻣ ﺔﺛﻼﺛ ﻲﻓ ﺱﺎﻴﻛﻷﺍ ﺩﺪﻌﺘﻣ ﺾﻴﺒﻤﻟﺍ ﺔﻣﺯﻼﺘﻤﺑ ﺔﻀﻳﺮﻣ 60 ﻝ ﻲﻄﻋﺃ ﺝﻼﻌﻟﺍ ﻦﻣ ﺮﻬﺷﺃ ﺔﺛﻼﺛ.<br />
ﺎﻀﻳﺃ<br />
ﺔﻋﺮﺠﺑﻭ ﻦﻴﻣﺭﻮﻔﺘﻤﻟﺍ ﺭﺎﻘﻌﺑ ﻦﺠﻟﻮﻋ ﺔﻀﻳﺮﻣ 20 ﻦﻣ ﺔﻧﻮﻜﻣ ﺔﻴﻧﺎﺜﻟﺍ ﺔﻋﻮﻤﺠﻤﻟﺍ ﻭ ﺎﻴﻣﻮﻳ ﻢﻐﻠﻣ 750 ﺔﻋﺮﺠﺑ ﻦﻳﺭﺎﻤﻴﻠﻴﺴ ﻟﺍ ﺭﺎﻘﻌﺑ ﻦﺠﻟﻮﻋ ﺔﻀﻳﺮﻣ<br />
ﻦﻳﺭﺎﻤﻠﻴﺴﻟﺍﻭ ﺎﻴﻣﻮﻳ ﻢﻐﻠﻣ 1500 ﻦﻴﻣﺭﻮﻔﺘﻴﻤﻟﺍ ﺞﻳﺰﻤﺑ ﻦﺠﻟﻮﻋ ﺎﻀﻳﺃ ﺔﻀﻳﺮﻣ 20 ﻦﻣ ﺔﻧﻮﻜﻤﻟﺍﻭ ﺔﺜﻟﺎﺜﻟﺍ ﺔﻋﻮﻤﺠﻤﻟﺍ ﺎﻤﻨﻴﺑ ﺎﻴﻣﻮﻳ ﻢﻐﻠﻣ 1500<br />
ﻥﺍ.<br />
ﺝﻼﻌﻟﺍ ﺔﻳﺎﻬﻧ ﺪﻌﺑ ﻞﻴﻟﺎﺤﺘﻟﺍ ﻞﻜﻟ ﻯﻮﺘﺴﻤﻟﺍ ﻦﺴﺤﺗ ﺕﺮﻬﻇﺃ ﺞﺋﺎﺘﻨﻟﺍ.<br />
ﺔﺋﺰﺠﻣ ﻉﺮﺠﺑ ﺝﻼﻌﻟﺍ ﺕﺬﺧﺍ ﻊﻴﻣﺎﺠﻤﻟﺍ ﻩﺬﻫ ﻞﻛ.<br />
ﺎﻴﻣﻮﻳ ﻢﻐﻠﻣ 750 ﺔﻋﺮﺠﺑ<br />
ﻦﻳﺭﺎﻤﻴﻠﻴﺴﻟﺍ ﺞﻳﺰﻤﺑ ﻦﺠﻟﻮﻋ ﻲﺗﺍﻮﻠﻟﺍ ﺕﺎﻀﻳﺮﻤﻟﺍ ﺔﻋﻮﻤﺠﻣ ﻲﻓ ﻞﻀﻓﺃ ﺖﻧﺎﻛ ﺔﺿﺎﺑﻻﺍ ﺔﺒﺴﻧﻭ ﻥﻭﺮﻴﺘﺴﻴﺟﻭﺮﺒﻟﺍ ﺕﺎﻳﻮﺘﺴﻣ ﺔﺒﺴﻧ ﺓﺩﺎﻳﺯ<br />
ﺮﻴﺛﺄﺗ ﻪﻟ ﺱﺎﻴﻛﻷﺍ ﺩﺪﻌﺘﻣ ﺾﻴﺒﻤﻟﺍ ﺔﻣﺯﻼﺘﻤﺑ ﺕﺎﺑﺎﺼﻤﻟﺍ ﺕﺎﻀﻳﺮﻤﻟﺍ ﺝﻼﻌﻟ ﻦﻴﻣﺭﻮﻔﺘﻤﻠﻟ ﻦﻳﺭﺎﻤﻴﻠﻴﺴﻟﺍ ﺔﻓﺎﺿﺇ ﻥﺇ ﺝﺎﺘﻨﺘﺳﻻﺍ ﻲﻓﻭ . ﻦﻴﻣﺭﻮﻔﺘﻴﻤﻟﺍ ﻭ<br />
. ﺔﺿﺎﺑﻻﺍ ﺔﺒﺴﻧﻭ ﺔﺑﺮﻄﻀﻤﻟﺍ ﺕﺎﻧﻮﻣﻮﻬﻟﺍ ﻰﻠﻋ ﻦﺴﺣ<br />
Introduction<br />
Polycystic ovary syndrome (PCOS) is a<br />
heterogeneous disorder of uncertain aetiology;<br />
it is the most common endocrinopathy in women<br />
and most common cause of anovulatory infertility,affecting<br />
5-10% of population of reproductive<br />
age. (1) It is characterized by chronic<br />
anovulation and hyperandrogenism. (2). Insulin<br />
resistance and associated hyperinsulinemia also<br />
have been recognized as important pathogenic<br />
factors in determining the majority of PCOS<br />
women particularly when obesity is present . (3)<br />
Most but no t all women with PCOS have hyperinsulinemia<br />
with insulin resistance (4) .The association<br />
between hyperinsulinemic insulin resis-<br />
1Corresponding author E- mail : Mohammed_taher34@yahoo.com<br />
Received : 16/1/2010<br />
Accepted 26/5/2010<br />
11<br />
tance and PCOS well recognized and play an<br />
import role in the development of<br />
PCOS (5) .Hyperinsulinemia has been shown to<br />
reduce sex hormone binding globuline (SHBG)<br />
synthesis in liver (6) and insulin has a direct effect<br />
on ovarian steroidogenesis in theca cell. (7)<br />
Metformin is the oldest and still most insulin<br />
sensitizer world wide in the treatment o f type2<br />
diabetes mellitus and PCOS-associated with<br />
insulin resistance. It is a biguanide derivative<br />
and considered as an insulin sensitizer since it<br />
lowers glucose levels without increasing insulin<br />
secretion . (8)
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
Silymarin is an active polyphenolic flavenoid<br />
extracted from fruits(seeds) of medicinal plant<br />
silybum marianum (milk thistle), extracts were<br />
standardized to contain 70-80% silymarin complex<br />
which comprised mainly of three major<br />
flavolignans , silybinin silychristin and silydianin<br />
of which silybinin is the most biological<br />
active. Silymarin is considered to be very safe<br />
and there are only few reports on its adverse<br />
effects,mainly a mild laxative effect has been<br />
observed in occasional instances and there are<br />
no known contraindications or side effects reported<br />
during its regular use. (9) According to the<br />
multiple pharmacological actions of silymarin,<br />
silybinin have been clinically evaluated in diabetics<br />
for their therapeutics value reduces the<br />
lipoperoxidation of cell membrane and insulin<br />
resistance significantly, decreasing endogenous<br />
insulin overproduction and the need for exogenous<br />
insulin administration. (10) So this study<br />
was designed to evaluate the efficacy of silymarin<br />
as insulin sensitizer improving an ovulation<br />
rate by treatment of PCOS and consequently<br />
its effect on hormonal and biochemical profile<br />
of the patients and comparing it with a classical<br />
one, metformin.<br />
Materials and Methods<br />
Patients<br />
This study was conducted into Baghdad<br />
city ,in al-Elwia maternity teaching hospital<br />
from 12/2006-6/2007.The study groups included<br />
80 women selected randomly, 60 patients<br />
with PCOS aged (19-39) years with a<br />
mean age ( 27.5) years and 20 healthy control<br />
women aged (21-32) years with mean age (24)<br />
years.The diagnosis of PCOS was made by the<br />
gynaecologists depending on ultrasound examination<br />
,clinical features and laboratory tests<br />
according to diagnosis criteria of ( Rotterdam<br />
2003) (11) . Table-1 shows that the clinical presentations<br />
of patients in present study like those<br />
reported in other studies of polycystic ovary<br />
syndrome in that it is a heterogeneous disorder<br />
Investigations included : serum fasting glucose<br />
levels, fasting insulin levels, serum testosterone,<br />
serum progesterone and serum leutinizing hormone<br />
(LH).All patients participitated in this<br />
study were diagnosed having PCOS and were<br />
non-diabetic, not hypertensive, not pregnant ,<br />
and not taking any medications that affect the<br />
reproductive or metabolic functions with 90<br />
days of study. The patients were followed<br />
weekly regularly under gynecologist supervision<br />
during the period of treatment.The women<br />
were grouped into 4 groups as follow:<br />
Group 1: included 20 PCOS patients ,with BMI<br />
(31.22±1.138 Kg/m2),and age (19-31) years.<br />
They received Sylimarin tablets (750mg/day) in<br />
3 divided doses after meals for 3 months.<br />
12<br />
Group 2: included 20 patients with BMI<br />
30.84±1.23kg/m2) and age (20-35) years. The<br />
treatment was including metformin tablets<br />
1500mg/day in 3 divided doses (500mg after<br />
meals for 3 moths.<br />
Group 3: included 20 patients with BMI<br />
32.83±1.37 kg/m2), age (22-39) years. The<br />
treatment was consisting of combination of 2<br />
drugs (sylimarin 750 mg/day ) and metformin (<br />
1500 mg /day) in 3 divided doses for 3 months.<br />
Group 4: included 20 healthy women with<br />
BMI 28.4±1.01kg/m2) ,age (21-32) years and<br />
these women were with regular cycle (21-32<br />
days) who were taken fro m outside of the hospital<br />
and selected as controls.<br />
Sample collection<br />
Venous blood sample withdrew after<br />
overnight fasting ( at least 12 hours of fasting )<br />
fro m PCOS women and the control group . For<br />
the subjects with regular cycles ,the samples<br />
were taken at 3-5 days after the cycle for determination<br />
of serum LH and the sample for<br />
progesterone were taken at 21 days of the cycle<br />
.The other patients with irregular cycle ,the<br />
samples were taken randomly. The samples<br />
were taken from the patients before starting<br />
and after one month of treatment .<br />
Biochemical analysis<br />
Determination of serum glucose and insulin<br />
levels<br />
Fasting serum glucose and insulin levels<br />
were measured by commercial kit obtained<br />
fro m Randox using enzymatic method (12,13) .<br />
Determination of Homeostasis Model Assessment<br />
of insulin Resistance (HOMA-IR) ,<br />
HOMA - IR was calculated using the<br />
following formula (14) :<br />
HOMA-IR=Fasting glucose (mmol/L)× Fasting<br />
insulin (pmol/ml)/22.5.<br />
Insulin resistance patients were defined as having<br />
HOMA>2.7.<br />
Determination of serum testosterone (15) and<br />
LH levels (16) :<br />
Serum testosterone and LH levels were<br />
determined by radioimmunoassay(RIA) method<br />
using a kit provided by Sigma-Aldrich.<br />
Determination of serum progesterone & Ovulation<br />
Rate<br />
Serum progesterone levels were determined<br />
using kit obtained from Sigma-Aldrich ,<br />
using (RIA) method, and the ovulation rate was<br />
determined according to mid-luteal phase<br />
progesterone level that was equal to or more<br />
than 16nmol/L (5ng/ml) . (17)<br />
Determination of body mass index(BMI)<br />
BMI was calculated using standard<br />
formula : BMI= weight (kg)/high (m2).
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
Obese patients were defined as having MBI ><br />
27kg/m2 (18).<br />
Ultrasound study<br />
Transvaginal ultrasound study scan is<br />
performed for each patient at about day 12 of<br />
the cycle in order to to confirm follicular<br />
changes that appear through biochemical and<br />
hormonal changes , also it was repeated for<br />
each patient who had serum progesterone levels<br />
higher than or equal to 16nmol/L in order to<br />
confirm improvement of fertility and response<br />
of patients to treatment and follow up follicular<br />
development . (19)<br />
Diagnosis<br />
Hyperandrogenism<br />
Based on criteria of Androgen Excess<br />
Society (AES 2006), which recommended the<br />
following diagnostic criteria for PCOS hyperandrogenemia.<br />
(20)<br />
1. Hyperandrogenism ( hirsutism and /or<br />
hyperandrogenemia )<br />
2. Ovarian dysfunction (oligo-anovulation<br />
and /or PCOS).<br />
3. Exclusion of related disorders such as<br />
hyperprolactenemia and congenital adrenal<br />
hyperplasia.<br />
Hirsutism<br />
Based on Ferriman-Gallwey score , evaluates<br />
nine body sites including the face, chest ,<br />
areolae , linea alba , upper back, lower back,<br />
buttocks, inner thighs and external genetalia . (21)<br />
Infertility<br />
Inability of any couple to conceive a child<br />
within a 12 months period of unprotected coitus<br />
( sexual intercourse) . (22)<br />
Statical analysis<br />
Student t-test was used to examine the<br />
quantitative differences in the mean parameters.<br />
The results are expressed as mean±SD and the<br />
P-values
Groups<br />
1<br />
2<br />
3<br />
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
Table 2: Effect of me tformin and /or silymarin on Insulin ,glucose ,HOMA-IR ratio, total testosterone<br />
and proge sterone in wo men with PCOS.<br />
Analyte s Control Base line Afte r 1 M Afte r 2M After 3M<br />
Insulin(pmol/L) 57.5±0.359 92.18±4.73 89.35±0.35* 85.65±4.28* 81.44±3.66*<br />
Glucose(mg/dl) 5.1±0.17 5.29± 0.29a 5.01± 0.192NS 4.88±0.128* 4.73±0.128*<br />
HOMA 2.13±0.015 3.11± 0.244a 2.865±0.233* 2.673±0.178* 2.02±0.178*<br />
Testosterone(nmol/L) 1.45±0.03 4.59± 0,223a 4.427±0.242* 4.242±0.303* 3.396±0,318<br />
Progesterone(nmol/L) 17.15±0,02 12.84±0,612a 13.39±0.682NS 13.96±0.804* 14.41±0.942*<br />
LH(u/L) 5.2±0.365 9.38± 0.317a 9.18± 0.284NS 9±0.245* 8.71±0,376*<br />
Insulin(pmol/L) 57.5±0.359 83.7±4.49a 82.1±3.468 80.8±3.01* 74.5±4.73*<br />
Glucose(mg/dl) 5.1±0.17 5.35± 0.362a 4.49± 0.209* 4.63±0.35* 4.25±0.229*<br />
HOMA 2.13±0.015 2.68± 0.226a 2.59± 0.212* 2.39±0.199* 2.02±0.178*<br />
Testosterone(nmol/L) 1.45±0.03 4.07± 0.199a 3.938±0.213* 3.765±0.185 3.9±0.167*<br />
Progesterone(nmol/L) 17.15±0,02 12.95±0.967a 13.517±0.941* 14.04±1.01* 15.01±1.33*<br />
LH(u/L) 5.2±0.365 111.13±0.87a 10.56±0.839NS 10.10±0.644* 9.52±0.741*<br />
Insulin(pmol/L) 57.5±0.359 106±6.6 94.05±4.26* 84.16±4.50* 77.22±3.09<br />
Glucose(mg/dl) 5.1±0.17 5.12±0.301a 4.58±0.330* 4.27±0.369* 3.87±0.22*<br />
HOMA 2.13±0.015 3.55±0.172a 2.75±0.144* 2.298±0.245* 1.91±0.135*<br />
Testosterone(nmol/L) 1.45±0.03 4.59±0.942a 4.18±0.176* 4.06±0.159* 3.9±0.167*<br />
Progesterone(nmol/L) 17.15±0,02 13.88±0.875a 14.46±0.792* 15.10±0.673* 16.31±0.916*<br />
LH(u/L) 5.2±0.365 10.08±0.510a 9.33±0.480* 8.89±4.22* 8.54±0.515*<br />
Values are Mean±SD , a P< 0.05 for comparison with control group ,*P0.05<br />
Table 3: Comparison among me an % of increame<br />
nt in progesterone levels (nmol/L) and<br />
number of women who had ovulate d during<br />
the study in a ll groups of PCOS patients.<br />
Group1<br />
(sylimarin)<br />
Group2<br />
( Metformin)<br />
Group 3<br />
(combination)<br />
%<br />
of 1 st<br />
Month<br />
%<br />
of 2 nd<br />
Month<br />
%<br />
of 3 rd<br />
Month<br />
No.of<br />
wome n<br />
ovulated<br />
4.28 8.72 12.22 4<br />
4.324 8.42 15.9 5<br />
4.179 8.79 17.51 10<br />
Discussion<br />
The percentage of patients with hirsutism<br />
and acne was 43.3% and 36% respectively (table-1)<br />
and this finding was consistence with<br />
other study performed in diagnosis of<br />
PCOS.Cutaneous manifestations of hyperandrogenism<br />
in PCOS include hirsutism,acne or<br />
14<br />
combination , and male –pattern hair loss ( androgenic<br />
alopecia); whereas acanthosis nigrigans<br />
is a cutaneous marker of hyperinsulinemia<br />
. (23) The study demonstrated that percentage of<br />
obese patients was 68.6% while it was 31.6%<br />
for lean , this is common in PCOS and it is in<br />
line with other studies which demonstrated that<br />
40-60% of women with PCOS are obese<br />
(BMI>27 kg/m2). (24,25) The present study<br />
showed that (51.6%) of the patients were infertile<br />
, 31.6% with amenorrhea, 56% with oligomenorrhea<br />
,11.6% with regular cycle,78.3%<br />
with insulin resistance and 85% with hyperandrogenemia<br />
,these results are in agreement partly<br />
with other results which demonstrate the<br />
presence of infertility by (55-75%) , amenorrhea<br />
(26-15%) ,oligomenorrhea (50-90%) regular<br />
cycle (22%) and hirsutism (60-90%) in<br />
women with PCOS . (24,26) The high levels of<br />
androgens lead to chronic anovulation , menstrual<br />
disturbances and hirsutism. PCOS patients<br />
typically have elevated LH levels and<br />
LH:FSH ratios. (27) because hyperandrogenism<br />
leads to abnormal folliculogenesis and endome-
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
trial development . (28,29) Hyperandrogenemia is<br />
a key feature of the syndrome; but it is not always<br />
linked to hyperandrogenic symptoms such<br />
as acne or hirsutism; indeed ,ethnic groups such<br />
as Asian shown insulin resistance and associated<br />
hyperinsulinemia are also now recognized<br />
as important pathogenic factors in determining<br />
hyperandrogenism in the majority of<br />
PCOS women ,particularly when obesity is<br />
present . (30) The present study illustrated a significant<br />
(P
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
rone and LH concentration. (41) Therefore it is<br />
probaple that effect of silymarin on progesterone<br />
levels were consequences of its effect on<br />
insulin resistance and hyprinsulinemia. There<br />
was remarkable response to combination treatment<br />
because each drug act by its own mechanism<br />
and higher increment in progesterone and<br />
ovulation rate exerted by each drug alone may<br />
be enhanced by their combination . The base<br />
line LH levels in this work increases significantly<br />
(P
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
reisstance &beta cell fuction from fasting<br />
plasma glucose &insulin concentration in<br />
man.Diabetolgia.1985;28:412-419.<br />
15. Ratccliffe WA, Corrie JE Dalziel et<br />
al.Clinical chem..J.1982;28:1341-1318.As<br />
cited by sigma-Aldrich Diagnostic.<br />
16. ThomasCM,Segers MF.Clin<br />
Chem.Apr.1988;34:768.As cited by Sigma-<br />
Aldrich Diagnostic.<br />
17. Yilmas S,Unaly Y, et al.The effect of metformin<br />
on insulin resistance ,clomipheninduced<br />
ovulation &pregnancy rate in<br />
women with polycystic ovary syndrome<br />
.European<br />
J.Obst.&Gynecol,&Reprod.Biology.2004;1<br />
13:214-220.<br />
18. Peter n.Herbert.Eating disorders.In: Andeoli,Carpenter<br />
(editor).Cecil essentials of<br />
medicine.Saunders Company,5 th end. 2001,<br />
515-521.<br />
19. Yeh HC,Futterweit W,Thornton JC. Polycystic<br />
ovarian disease:US features in 140<br />
patients.Radiology.19987;163:111-116.<br />
20. TaskForce on phenotype of PCOS of the<br />
Androgen Excess Society.Psition statement:The<br />
androgen Excess Society evidence-based<br />
criteria for defining PCOS as<br />
apredominantly hyperandrogenic syndrome.J.clinical<br />
Endeocr Meta 2006;91:<br />
9237-9245.<br />
21. Ferriman D &Gallway J.D. Clincal assessment<br />
of body hair growth in women.J Clincal<br />
Endocr Meta.1961;21:1440-1447.<br />
22. Text book of gynecology,L. Copel and,<br />
W.B.Saunders Co.,1993.<br />
23. Tsilchorozidou T,Overton C, Conway<br />
GS.The pathophysiology of polycystic<br />
ovary syndrome.Clin Endocrinol (0xf).<br />
2004;60:1-17.<br />
24. Futterweit W.Polycystic ovary syndrome<br />
clinical perspective&management.Ostet<br />
Gynecol Surv.1999;54:402-413.<br />
25. Campbell PJ, Geriich JE.Impact of obesity<br />
on isulin action in volunteers with normal<br />
glucose tolerance demonstration of a threshold<br />
for the adverse effect of obesity.J<br />
Clin Endocrnol Metabb.1990;70:1114-<br />
1118.<br />
26. Aziz R,Dunaif A,Giudice LC, et<br />
al.Diagnosis & management of polycystic<br />
ovary syndrome.Contemp Obstet Gynecol.1998;43(supp11):1-29.<br />
27. Legro RS,Kunselman AR,Dodson WC,et<br />
al.Prevenlance and predictors of risk for<br />
type 2 diabetes mellitus and impaired glucose<br />
tolerance in polycystic ovary syndrome<br />
:a prospective, controlled study in<br />
254 affected women.J clin Endocrinol Metab.1999;84:165-169.<br />
17<br />
28. Robert D.Utiger.Insulin &the polycystic<br />
ovary syndrome.N Engl J Med.1996;<br />
9(335):657-658.<br />
29. Franks S.Polylstic ovary syndrome.N.Eng J<br />
Med. 1989;333:853-861.<br />
30. Clifford K,Rai R,Watson H, et al. An informative<br />
protocol for the investigation of<br />
recurrent miscarriage:preliminary experience<br />
of 500 consecutive cases.Hum Reprod.1994;9:1328-1332.<br />
31. Laure C.Morin-Papunen,1lka Vauhkonen,<br />
et al.Insulin sensitivity ,insulin secretion &<br />
metabolic & hormonal parameters in<br />
healthy women & women with PCOS.<br />
Human Reproduction.2000; 15(6): 1266-<br />
1274.<br />
32. Landian K,Tengborn L,Smith U.Metformin<br />
and metoprolol CR treatment in non-obese<br />
men.J Intern Med.1994;235:335-341.<br />
33. Hundal RS&Inzucchi SE.Metformin : new<br />
understanding, new uses. Drugs. 2003; 63:<br />
1879-1894.<br />
34. 4)Soto C, Recoba R,Barron H et al. Silymarin<br />
increases antioxidant enzymes in alloxan-induced<br />
diabetes in rat pancrease.Comp<br />
Biochem Physiol Toxicol<br />
Pharmacol.2003;136:205-212.<br />
35. Maddux BA,See W, et al. Protection<br />
against oxidative stress-induced insulin resistance<br />
in rat L6 muscle cells by micro<br />
molar concentrations of alpha-lipoic acid.Diabetes<br />
. 2001;50:404-410.<br />
36. Gonzalez F, Thusu K, abdel-Rahman E, et<br />
al. Elevated serum levels of tumor necrosis<br />
factor in normal –eight women with polycystic<br />
ovary syndrome.Metabolism. 1999;<br />
48:437-441.<br />
37. Conway GS , Honour JW,Jacobs HS. Heterogenety<br />
of polycystic ovary syndrome:<br />
clinical , endocrine and ultrasound feaures<br />
in 556 patients.Clin Endocrinol (Oxf)<br />
1989;30:459-470.<br />
38. Valezquezz EM, Mendoza S, Hamer T, et<br />
al. Metformin therapy in PCOS reduces<br />
hyperinsulinemia , insulin resistancd,<br />
hyperandrogenemia , and systolic blood<br />
pressure , while facilitating normal menses<br />
and pregnancy.Metabolism. 1996;43:647-<br />
654.<br />
39. Acbay O, Gundogdu S. Can metformin<br />
resuce insulin reistance in polycystic ovary<br />
syndrome? Fertil Steril.1996;65:946-949.<br />
40. Velazqquez E,Acosta A, Mendoza SG.<br />
Menstrual cylicity after metformin therapy<br />
in PCOS.Obsest Gynecol.1997;90:392-395.<br />
41. Meenakumari KJ,Agarwal S, Krishna A, et<br />
al. Effect of metformin in treatment of luteal<br />
phase progesterone concentration in<br />
PCOS.Brazilin J Medical & Biological Research<br />
.2004;37:1637-1644.
Iraqi J Pharm Sci, Vol.19(2) 2010 Polycystic ovary syndrome and silymarin<br />
42. Derelli D, Derelli T, Bayraktar F, et al.<br />
Endocrine & metabolic effects of rosiglitazone<br />
in non- obese women with<br />
PCOS,Endocrinol J.2005;52(3):299-308.<br />
43. Karlo BN, Loucks TL, Begra<br />
SL.Neuromodulation in polycystic ovary<br />
syndrome.Obset Gynecol Clin North<br />
Am.2001; 28:35-62.<br />
44. Frank S: polycystic ovary syndrome . N<br />
Engl J Med. 1989; 333: 853-861.<br />
18<br />
45. Waldstreicher J, Santoro NF, Hall<br />
JE,Filicari M, Crowley WF<br />
Jr.Hyperfunction of the hypothalamicpituitary<br />
axis in women with polycystic<br />
ovarian disease.Idirect evidence for partal<br />
gonadotrophin desensitization . J Clin Endocrinol<br />
Metab . 1988; 66:165-172.<br />
46. Vincenzo De Leo, Antonio La Marca and<br />
Felice petraglia. Insulin lowering agents in<br />
the management of polycystic ovary syndrome<br />
.2003;24(5):633-667.
Iraqi J Pharm Sci, Vol.19(2) 2010 Study proteins profile with β–thalassemia<br />
A Comparative Biochemical Study of Proteins Profile in Iraqi<br />
Children and Adolescent with β–Thalassemia<br />
Ali M. Malik* , Emad M. Malik**, Nawal MJ Al-Shammaa*** and<br />
Zeinab M. Al-Rubaei***<br />
*Central Children Hospital,Ministry of Health,Baghdad,Iraq .<br />
**Departement of Pharmaceutical Chemistry,Risafa Directurate,Ministry of Health,Baghdad,Iraq .<br />
*** Department of Che mistry,College of Education , University of Baghdad ,Baghdad,Iraq .<br />
Abstract<br />
The aim of the present research is to study different protein fractions in sera of children and<br />
adolescent with β –thalassemia major and minor and to compare the results with that of healthy<br />
control.One hundred fifty children and adolescents were enrolled in this study,including 50 patients<br />
with β- thalassemia major , 50 patients with β- thalassemia minor as pathological control group and<br />
another apparently 50 healthy individuals as a control group. The age of all studied groups ranged<br />
from (4-18)years.Total protein, albumin and immunoglobulins were estimated in sera of all subjects. A<br />
Significant decrease was found in the total protein and albumin levels in sera of both major and<br />
minor thalassemic patients compared to normal groups. A Significant increase in immunoglobulin<br />
levels (IgG, IgM and IgA) was found in the sera of major and minor β-thalassemia patients compared<br />
to control.Different protein parts in sera of all subjects were detected using cellulose acetate<br />
electrophoresis.The results revealed significant reduction in β- globulin fractions in β- thalassemia<br />
major patients compared to the normal and pathological control groups. Significant elevations in γ-<br />
globulins fractions were observed in both major β- thalassemia and minor β- thalassemia as compared<br />
to normal control group. As a Conclusion the alteration in some protein parts occurred which is more<br />
obvious in major thalassemia patients compared to the normal and pathological control groups.<br />
Key words: Protein Parts , Elecrtrophoresis , β-Thalassemia.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﻂـﺳﻮﺘﻤﻟﺍ ﺾﻴـﺑﻻﺍ ﺮـﺤﺒﻟﺍ ﻡﺩ ﺮـﻘﻓ ﺽﺮﻤﺑ ﺎﺑﺎﺼﻣ ﻢﻬﻨﻣ ﻥﻮﺴﻤﺧ ٬ ﻕﺍﺮﻌﻟﺍ ﻲﻓ ﺭﺎﻐﺼﻟﺍ ﻦﻣ"<br />
ﺎﺼﺨﺷ 150 ﻦﻣ ﻡﺪﻟﺍ ﺝﺩﺎﻤﻧ ﺖﻌﻤﺟ<br />
50ﻭ<br />
٬ ﺔﻴـﺿﺮﻣ ﺓﺮﻄﻴـﺳ ﺔـﻋﻮﻤﺠﻤﻛ ﺍﻭﺮﺒﺘﻋﺍ ﻦﻳﺬﻟﺍ ﻭ ﻒﻴﻔﺨﻟﺍ ﻂﺳﻮﺘﻤﻟﺍ ﺾﻴﺑﻻﺍ ﺮﺤﺒﻟﺍ ﻡﺩ ﺮﻘﻓ ﺽﺮﻤﺑ ﺎﺑﺎﺼﻣ ﻢﻬﻨﻣ ﻥﻮﺴﻤﺧﻭ ٬ﺪﻳﺪﺸﻟﺍ<br />
ﻒﻠﺘﺨﻣ ﺔﺳﺍﺭﺩ ﺚﺤﺒﻟﺍ ﻦﻣ ﻑﺪﻬﻟﺍ . ﺔﻨﺳ(<br />
184<br />
) ﻦﻴﺑ ﺔﺳﻭﺭﺪﻤﻟﺍ ﻊﻴﻣﺎﺠﻤﻟﺍ ﺭﺎﻤﻋﺍ ﺡﻭﺍﺮﺘﺗ.<br />
ﺔﻴﻌﻴﺒﻃ ﺓﺮﻄﻴﺳ ﺔﻋﻮﻤﺠﻤﻛ ءﺎﺤﺻﻻﺍ ﻦﻣ ﺎﺼﺨﺷ<br />
ﺔـﻋﻮﻤﺠﻤﻟﺍ ﻊـﻣ ﺎـﻬﺘﻧﺭﺎﻘﻣﻭ ﻕﺍﺮـﻌﻟﺍ ﻲـﻓ ﺭﺎﻐـﺼ ﻟﺍ ﺹﺎﺨـﺷﻻﺍ ﻦﻣ ﺎﺘﻴﺑ ﻉﻮﻧ ﻂﺳﻮﺘﻤﻟﺍ ﺾﻴﺑﻻﺍ ﺮﺤﺒﻟﺍ ﻡﺩ ﺮﻘﻓ ﻰﺿﺮﻣ ﻲﻓ ﻦﻴﺗﻭﺮﺒﻟﺍ ءﺍﺰﺟﺍ<br />
ﺕﺭﺎـﺷﺍ . ﺔﻄﺑﺎﻀﻟﺍﻭ ﺔﻴﺿﺮﻤﻟﺍ ﻊﻴﻣﺎﺠﻤﻟﺍ ﻦﻣ ﻞﻛ ﻞﺼﻣ ﻲﻓ ﺕﺎﻨﻴﻟﻮﻴﺑﻮﻠﻛﻮﻨﻴﻣﻻﺍﻭ ﻦﻴﻣﻮﺒﻟﻻﺍﻭ ﻲﻠﻜﻟﺍ ﻦﻴﺗﻭﺮﺒﻟﺍ ﻦﻣ ﻞﻛ ﺱﺎﻴﻗ ﻢﺗ . ﺔﻄﺑﺎﻀﻟﺍ<br />
) ﺕﺎﻨﻴﻟﻮﻴﺑﻮﻠﻛﻮ ﻨﻴﻣﻻﺍ ﻦﻣ ﻞﻛ ﻯﻮﺘﺴﻣ ﻲﻓ ﺔﺤﺿﺍﻭ ﺓﺩﺎﻳﺯ ﻊﻣ ﻦﻴﻣﻮﺒﻟﻻﺍﻭ ﻲﻠﻜﻟﺍ ﻦﻴﺗﻭﺮﺒﻟﺍ ﻯﻮﺘﺴﻣ ﻲﻓ ﻱﻮﻨﻌﻣ ﻥﺎﺼﻘﻧ ﺩﻮﺟﻭ ﻰﻟﺍ ﺞﺋﺎﺘﻨﻟﺍ<br />
ﻲﻓ ﻦﻴﺗﻭﺮﺒﻟﺍ ءﺍﺰﺟﺍ ﺔﺳﺍﺭﺩ ﻢﺗ . ﺔﻴﻌﻴﺒﻄﻟﺍ ﺔﻄﺑﺎﻀﻟﺍ ﺔﻋﻮﻤﺠﻤﻟﺍ ﻊﻣ ﺔﻧﺭﺎﻘﻣ ﺔﺳﻭﺭﺪﻤﻟﺍ ﻰﺿﺮﻤﻟﺍ ﻊﻴﻣﺎﺠﻣ ﻦﻣ ﻞﻛ ﻲﻓ(<br />
IgAﻭ<br />
IgGﻭ<br />
IgM<br />
ﻡﺪـﻋ ﻰﻟﺍ ﺞﺋﺎﺘﻨﻟﺍ ﺕﺭﺎﺷﺍ ﻭ ﻲﺋﺎﺑﺮﻬﻜﻟﺍ ﻞﻴﺣﺮﺘﻟﺍ ﺔﻴﻨﻘﺘﺑ ﺔﻴﻌﻴﺒﻄﻟﺍ ﺔﻄﺑﺎﻀﻟﺍ ﺔﻋﻮﻤﺠﻤﻟﺍ ﻭ ﻂﺳﻮﺘﻤﻟﺍ ﺾﻴﺑﻻﺍ ﺮﺤﺒﻟﺍ ﻡﺪﻟﺍ ﺮﻘﻓ ﻰﺿﺮﻣ ﻝﻮﺼﻣ<br />
ﻰﺿﺮﻤﻟﺍ ﻊﻴﻣﺎﺠﻣ ﻦﻴﺑ ﺎﻣﺎﻛ ﻦﻴﻟﻮﻴﺑﻮﻠﻛ<br />
ﻲﻓ ﺔﺤﺿﺍﻭ ﺓﺩﺎﻳﺯ ﻊﻣ ﺎﺘﻴﺑ ﻦﻴﻟﻮﻴﺑﻮﻠﻛ<br />
ﻲﻓ ﺢﺿﺍﻭ ﺺﻘﻧ ﻙﺎﻨﻫ ﺎﻤﻨﻴﺑ ﺎﻔﻟﺍ–<br />
ﻦﻴﻟﻮﻴﺑﻮﻠﻛ ﻲﻓ ﺺﻘﻧ ﺩﻮﺟﻭ<br />
ﺔﻧﺭﺎﻘﻣ ﺓﺪﻳﺪﺸﻟﺍ ﻂﺳﻮﺘﻤﻟﺍ ﺾﻴﺑﻻﺍﺮﺤﺒﻟﺍ ﻡﺩ ﺮﻘﻓ ﻰﺿﺮﻣ ﻲﻓ ﺔﺻﺎﺧﻭ ﺔﻳﻮﻨﻌﻣ ﻦﻴﺗﻭﺮﺒﻟﺍ ءﺍﺰﺟﺍ ﻲﻓ ﺮﻴﻐﺘﻟﺍ ﻥﺍ ﺝﺎﺘﻨﺘﺳﻻﺍ ﻢﺗ . ﺔﻔﻴﻔﺨﻟﺍﻭ ﺓﺪﻳﺪﺸﻟﺍ<br />
. ﺔﻴﻌﻴﺒﻄﻟﺍ ﺔﻄﺑﺎﻀﻟﺍ ﺔﻋﻮﻤﺠﻤﻟﺍﻭ ﻒﻴﻔﺨﻟﺍ ﻂﺳﻮﺘﻤﻟﺍ ﺾﻴﺑﻻﺍﺮﺤﺒﻟﺍ<br />
ﻡﺩ ﺮﻘﻓ ﻰﺿﺮﻣ ﻊﻣ<br />
Introduction<br />
Thalassemia is the name of a group of<br />
geneticlly (inherited), blood disorders , all of<br />
which involve under production of<br />
haemoglobin , and partial or complete failure<br />
of synthesis a specific type of globin chain.The<br />
defect may affect the α, γ and δ chain or may<br />
affect some combination of the β, γ and δ<br />
chains in the same patient, but never α and β<br />
chain together, unmatched globins could<br />
precipitate and damage RBC membranes<br />
causing their destruction while still in the<br />
marrow [1,2] .Beta (β)- thalassemia manifest<br />
clinically has three major groups: 1-βthalassemia<br />
major, 2- β- thalassemia<br />
1Corresponding author E- mail : Elaf95@yahoo.com<br />
Received : 11/1/2010<br />
Accepted : 2/6/2010<br />
19<br />
intermedia and 3-β- thalassemia minor<br />
(trait) [2] .β -thalassemia major occurs at a high<br />
gene frequency throughout the Mediterranean<br />
populations, the Middle East, India and<br />
Southern China through Thailand<br />
populations [3] .The prevalence of β–<br />
thalassemia in Iraq have not taken much<br />
intention in previous studies in spite of the<br />
large population affected by this<br />
haematological disease.Proteins are substances<br />
that made up of smaller building blocks called<br />
amino acids [3] , which are an important<br />
constituents of all cells and tissues. Human<br />
serum contains more than 125 well identified
Iraqi J Pharm Sci, Vol.19(2) 2010 Study proteins profile with β–thalassemia<br />
proteins. So there are many different kind of<br />
proteins in the body with many different<br />
functions,for the example:- enzymes, some<br />
hormones, hemoglobin, immunoglobulin<br />
(antibodies) [4] . The major sites of synthesis of<br />
plasma proteins are the liver and the immune<br />
system [5] . Total protein level depend on the<br />
balance between their synthesis and their<br />
catabolism or loss from body . A test for total<br />
serum protein measures total amount of protein<br />
in blood serum as the amounts of albumin and<br />
globulins [6] . Albumin has a single polypeptide<br />
chain of (580) amino acids. It is a very stable<br />
protein with a high net negative charge at the<br />
physiological pH. It has a molecular weight of<br />
(66)KDa . Albumin molecule could serve as<br />
hormones and various metabolites as well as<br />
drugs and antibiotics carrier. Albumin also<br />
functions in the maintenance of proper osmotic<br />
pressure [7] .The immunoglobulins which are<br />
antibodies, are a heterogeneous group of<br />
plasma proteins produced by B-<br />
lymphocytes . .These proteins are important in<br />
preventing and fighting infections. Elevation in<br />
the serum levels of immunoglobulin are seen<br />
in infectious diseases and thalassemia [8] .Many<br />
studies have been carried out to evaluate<br />
changes of the immune system in thalassemia<br />
patients, considering the humoral and cellular<br />
immune system; but no consistent defect in<br />
white cells or immune functions had been<br />
documented [8] .The aim of the present research<br />
is to study different protein fractions in sera of<br />
children and adolescent with β -thalassemia<br />
major and minor and to compare the results<br />
with that of healthy control.<br />
Materials and Methods<br />
Selection of subjects and blood sampling<br />
Six ml of venous blood sample was<br />
obtained from 150 children and adolescent<br />
attending Ibn Al-Baladi Hospital . 50 patients<br />
were with β -thalassemia major, 50 patients<br />
were with β- thalassemia minor (as<br />
pathological control group) and 50 apparently<br />
healthy individuals as control group.The age of<br />
all studied groups were ranging from (4-18)<br />
years.The blood samples were transferred into<br />
plain tubes, allowed to stand for 15 minutes at<br />
room temperature then centrifuged at 3500 rpm<br />
for (10) minutes. The resulting serum was<br />
separated and frozen at (-20 0 C) till used for<br />
the estimation of total ptotein (TP), albumin,<br />
IgM, IgA, IgG and performing electrophoresis<br />
for sera.<br />
Determination of Total Protein(TP)<br />
The concentration of total proteins was<br />
determined according to the colorimetric<br />
method described by Gornall A. [9-10] The<br />
20<br />
peptide bonds of proteins react with Cu 2+ in<br />
alkaline solution to form a colored complex in<br />
which the absorbance at 550 nm was<br />
proportional to the concentration of total<br />
protein in the specimen. The biuret reagent<br />
contains sodium potassium tartrate to complex<br />
cupric ions and maintains their solubility in<br />
alkaline solution .<br />
Determination of Albumin<br />
Albumin concentration in serum was<br />
measured using manual procedure, TECO<br />
diagnostics kit.Serum albumin binds<br />
selectively to the dye bromcresol green at the<br />
pH 4.2. The absorbances of the resulting<br />
albumin-dye complex, was read at 630 nm,<br />
was proportional to the albumin<br />
concentration [9] .<br />
Determination of IgM, IgA and IgG<br />
Immunoglobulins (IgM, IgA and IgG)<br />
were determined by using<br />
immunoturbidimetric<br />
method [11] .Immunoglobulins (IgM, IgA and<br />
IgG) form a complex with antibodies in<br />
solution. The absorbance of the complex could<br />
be measured spectrophotometrically at 340 nm.<br />
Immunoglobulin concentrations for each<br />
sample was estimated by the equation obtained<br />
from a comparable standard curve for each<br />
type .<br />
Serum Protein Electrophoresis<br />
Electrophoresis is referring to the<br />
transport of electrically charged particles in an<br />
electric field,that can be utilized for the<br />
characterization of their components after a<br />
comparison to references.To perform an<br />
electrophoretic separation requires the support<br />
material (cellulose acetate) which was made<br />
with buffer previously placed in the electrode<br />
chamber then sample (serum) was applied to<br />
the support, and electrophoresis was performed<br />
by conducting to electric power for 40 min,<br />
using a constant voltage (150 v). The support<br />
was then removed from the electrophoresis<br />
unit stained with 1% penceau S. After washing<br />
out excess dye, the support media was dried<br />
then the electrograms were scanned [9] .<br />
Statistical Analysis<br />
Data presented as means and standard<br />
deviation. Study of T-Test (p) was used to<br />
compare the significance of the difference in<br />
the mean values of any groups( p ≤ 0.05) were<br />
considered statistically significant. The overall<br />
predictive values for the results in all studied<br />
groups were performed according to<br />
[12] .<br />
biostatistics by Daniel in 1987
Iraqi J Pharm Sci, Vol.19(2) 2010 Study proteins profile with β–thalassemia<br />
Results and Discussion<br />
Table (1) summarizes the results of<br />
estimated levels of serum total protein (TP)<br />
,albumin ,globulin and (A/G) ratio expressed<br />
21<br />
as (mean ± SD) in sera of normal<br />
control,thalassemia minor and β- thalassemia<br />
major .<br />
Table 1 : Total protein (TP), albumin,globulin and (A/G) ratio le ve ls in se ra of β- thalasse mia<br />
major, minor thalassemia patients and normal control .<br />
TP Albumin Globulin (A/G)ratio t-Test<br />
Groups<br />
gm/dl gm/dl gm/dl<br />
(No.=50) (No.=50) (No.=50)<br />
thalassemia major 5.46± 0.54* 3.83± 0.54* 1.626± 0.011* 2.35 * P≤ 0.05<br />
(thalassemia minor) 6.88 ± 0.66* 4.55± 0.49* 2.335± 0.150* 1.94 * P≤ 0.05<br />
Control 7.99 ± 0.59 5.21 ± 0.48 2.775 ± 0.402 1.88 P≤ 0.05<br />
P significant difference from control values.<br />
*: significant difference between minor and major thalassemia patients.<br />
The (Mean ± SD) level of TP in sera of control<br />
group, and β- thalassemia patients (minor<br />
&major) in gm /dl were (7.99 ±0.59), (6.88 ±<br />
0.66) and (5.46 ± 0.54), respectively. The<br />
results showed a significant decrease of TP in<br />
β- thalassemia major and minor comparing<br />
with control. Also a significant decrease in TP<br />
of β- thalassemia major compared to minor<br />
was found . These results are in agreement<br />
with one study who claimed that the decrease<br />
in serum total protein is due to secondarily<br />
decreased synthesis of protein by the<br />
liver [13] .The results of serum albumin<br />
measurements revealed that the mean values of<br />
albumin in the three studied groups in gm/dl<br />
were (5.21 ± 0.48) , (4.55 ± 0.49) and (3.83 ±<br />
0.54) , respectively.The low levels of albumin<br />
may roughly be balanced by arise in<br />
immunoglobulin levels . This is quite common<br />
combination;where most of individual<br />
proteins , other than albumin , make relatively<br />
small contribution to total protein because of<br />
quite large percentage change in the<br />
concentration of one of them may not be<br />
detected as change in total protein so only<br />
low albumin levels are of clinical<br />
importance [14] .The (A/G)ratio of β- thalassemia<br />
patients (major &minor) were 2.35 and 1.94<br />
respectively decreased significantly to 1.88 in<br />
the control group. A ratio much less than one<br />
can give clues about problems in the<br />
body [15,16]. The serum levels of IgG, IgM and<br />
IgA in sera of control,minor and β- thalassemia<br />
major patients are shown in table (2).<br />
Furthermore,the results showed high levels of<br />
IgG, IgM and IgA in sera of thalassemia<br />
patients compared to control. Also a significant<br />
higher increase in immunoglobulins in sera of<br />
major thalassemic patients was noticed<br />
compared to thalassemia minor patients.<br />
Table 2 : Serum immunoglobulin le vels (IgA, IgM and IgG) of control, pathological control and<br />
β- thalassemia major patients.<br />
IgG<br />
IgM<br />
IgA<br />
Groups<br />
mg/dl mg/dl<br />
mg/dl t-Test<br />
(No.=50) (No.=50) (No.=50)<br />
thalassemia major patients 1519 ± 37.60* 220 ± 6.55* 241.6 ± 7.48* P≤ 0.05<br />
thalassemia minor Patients 1182 ± 18.41*<br />
Control 1004 ± 19.00<br />
148.68 ± 4.13* 202 ± 11.12*<br />
P significant difference from control values.<br />
* significant difference between minor and major thalassemia patients<br />
Such observations can be attributed to many<br />
factors. For instance repeated blood transfusion<br />
in β- thalassemia patients would result in a<br />
continuous exposure to various antigens and<br />
might lead to increased levels of serum<br />
immunoglobulins.Repeated infections also<br />
P≤ 0.05<br />
120.6 ± 3.04 168.6 ± 5.05 P≤ 0.05<br />
stimulate the immune system and may result<br />
in increased immunoglobulin levels [17,18] .<br />
Iron overload was suggested by some<br />
investigators as an important contributing<br />
factor in altering the immune parameters in<br />
thalassemia patients that results in increased
Iraqi J Pharm Sci, Vol.19(2) 2010 Study proteins profile with β–thalassemia<br />
migration of T helper cells to the gut and<br />
lymph nodes and this causes an increase in<br />
serum immunoglobulin levels in thalassemia<br />
patients [19,20] .Figure (1) showed sera<br />
electrophoresis pattern of thalassemia major<br />
,minor and control groups. Abnormal pattern<br />
of serum protein in sera of patients groups is<br />
obvious by the decrease shown in the albumin<br />
band and change in the pattern of β and γ<br />
globulins which include main proteins<br />
specially those of immunoglobulins.The<br />
decrease in albumin levels of patient groups<br />
could be due to decrease rate of synthesis ,or to<br />
an increase in catabolism rates which occurred<br />
Albumin<br />
Alfa-1 fraction<br />
Alfa-2 fraction<br />
Beta fraction<br />
Gamma fraction<br />
A<br />
+<br />
-<br />
A c c B<br />
Figure 1 : Serum prote in Electrophoresis Patte rn (c control)<br />
A- (le ft):Normal control and major β-tha lassemia patients<br />
B-(right):Normal control and patho logicalcontrol (minor)<br />
References<br />
1. Noguchi,C.,Butterwoth,J.,Karawajew,L.,K<br />
uppers,R.,Favaloro, F. ,and Jacobsohn , D. :<br />
Hematologica , 2004,89,1281-1283.<br />
2. Hope R., Longmore J., Mc Maunus S., and<br />
Wood A.: "xford Hand Book of Clinical<br />
Medicine"15 th ed.Oxford University<br />
Press,2001,22-25.<br />
3. Cappolini N., CohenA., and Proter<br />
J.:"Guidelines for the Clinical Monagement<br />
of Thalassemia". Thalassemia International<br />
Federaton,2000,1-29, 79-92.<br />
4. Lissaure T., and Colayden G.: "Illustration<br />
Text Book of Paeidiat rics",2 nd ed. , Mosby<br />
International Lmt.,2001,305.<br />
5. Kanieco J., Harvey J., and Bruss. M. :<br />
"Clinical Biochemistry of Domestic<br />
Animals " 5 th ed. , Academic Press. ,1990 ,<br />
120-226.<br />
22<br />
in many diseases [14] .Meanwhile results<br />
revealed a mild reduction in β globulin that<br />
may be due to the presence of transferrin<br />
(the iron bound protein)in this band,which is<br />
considered as to be the major component of<br />
the β-globulin fraction and appears as a distinct<br />
band on high resolution serum protein<br />
electrophoresis [22] . Also figure (1A) of<br />
electrophoresis showed a highly significant<br />
increase in γ- globulins in major β- thalassemia<br />
major patients compared to normal control,<br />
while figure (1B) showed a mild increase in γ-<br />
globulins in pathological control compared<br />
to normal control.The increase in γ-globulins<br />
related to hemoglobinopathy diseases [9] .<br />
6. Michael L., Janet L., and Edward P.:<br />
"Clinical Chemistry", 4 th ed. Lippincott<br />
Williams & Wilkins,2002,172.<br />
7. Nelson D. , Cox M. : "Lehninger Principles<br />
of Biochemistry" 3 rd ed. ,Worth Publishers<br />
, 2000 , 2, 877.<br />
8. Ahmad A., Susan J., Reza A., Soheila A.,<br />
Nima J.,MehranK.:Evalusion of serum<br />
levels of immunoglobulins in thalassemia<br />
patients,British Journal of Haematology ,<br />
2005,214: 220-223.<br />
9. Bishop M.,Pody E.,Schoff L.:"Clinical<br />
Chemistryprocedures , correlations ",5 th ed.,<br />
Lippincott Williams&Wilkins,2005,105-<br />
106, 194- 212.<br />
10. Gornall A.:Biolabo Regents ,biolabo.<br />
Manufacturer : Biolabo SA, Ref.<br />
8216,Maizy,France.
Iraqi J Pharm Sci, Vol.19(2) 2010 Study proteins profile with β–thalassemia<br />
11. Shivananda Nayak B.: "Manipal Manual of<br />
Clinical Biochemistry ", 3 rd ed., Medical<br />
publishers LTD., New Delhi,2007,96-100.<br />
12. Dainel W.: "Biostatestics: A Foundation<br />
for Analysis in the Health Science", 4 th<br />
ed.,1987, 127-139.<br />
13. Ismaail H.: Sialic acid and other<br />
biochemical parameters level in βthalassemia<br />
major child patients. M.<br />
Sc.Thesis College of Science.Al-<br />
Mustansiriyah University,2001..<br />
14. Zilva P.M., and Philip D. M.: "Clinical<br />
chemistry in Diagnosis and treatment'' 6 th<br />
ed.,2006,159-160.<br />
15. International myloma foundation : The<br />
Scientific and Medical Communties, 2008,<br />
818, 4487-7455.<br />
23<br />
16. 16-Dambro, Goldman:Cecil Textbook 0f<br />
Medicine,1 st ed,2000,300-309.<br />
17. Henk, S.,Leo,M. and Anneke ,B.:<br />
allontibodies after blood transfusions in a<br />
nonhematologic allommunized patients,<br />
Transfusion,2006,46(4),630-635.<br />
18. Weatherland D.,Clegg j.:"The Thalassemia<br />
Syndromes", 4 th ed.,London: Black well<br />
Scientific Publications,2000,120- 124.<br />
19. Weatherland D., Clegg J.,Higgs D., Wood<br />
W.:The hemoglobinopathies. In: The<br />
metabolic basis of inherited disease. 8 th ed.<br />
Mc Graw-Hill,2000,4000– 4656.<br />
20. Ranjna C.: "Practical Clinical<br />
Biochemistry ", 3 rd ed.,New Delhi,Medical<br />
publishers,2003,600-605.
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
Comparative Effects of Fentanyl, Medazolam, Lignocaine and<br />
Propranolol on Controlling the Hemodynamic Pressor Response<br />
during Laryngoscopy and Intubation<br />
May S. Al-Sabbagh* ,1<br />
*Department of Clinical Pharmacy, College of Pharmacy, University of Baghdad, Baghdad, Iraq.<br />
Abstract<br />
Laryngoscopy and tracheal intubation are considered the most invasive stimuli in anesthesia.<br />
They provoked cardiovascular responses that include hypertension, tachycardia and dysrhythmias.<br />
Various pharmacological approaches have been used to blunt or attenuate such pressor responses. The<br />
present study was designed to evaluate the effect of medazolom, lignocaine and propranolol as a<br />
valuable adjuvant to fentanyl in attenuating hemodynamic responses to endotracheal intubation in<br />
normotensive patients. Thirty two patient with physical status I or II according to the score of<br />
American Society of Anesthesiologist (ASA), scheduled for elective surgery under standard general<br />
anesthesia, were randomly allocated into four groups (8 patients in each group), assigned as F, M, L<br />
and P groups. Each patient in the four groups received 1 µg/kg i.v fentanyl. Patients in groups M, L<br />
and P are treated with 0.2 mg/kg i.v medazolam, 1.5mg/kg i.v lignocaine and 0.01mg/kg i.v<br />
propranolol respectively. Induction of anesthesia was then accomplished with 2mg/kg thiopental<br />
sodium followed by1.5mg/kg succinylcholine. Tracheal intubation was performed 2 minutes after<br />
induction of anesthesia. Heart rate, systolic blood pressure, diastolic blood pressure, mean arterial<br />
pressure and rate pressure product were measured before induction, after induction and at 2, 4, 6 and 8<br />
minutes after intubation. The results indicated no significant variation in the hemodynamic pressor<br />
response in all four groups with tracheal intubation. In conclusion, a minimum effective dose of i.v<br />
pre-medications (fentanyl, medazolom, lignocaine and propranolol) were found to be individually<br />
successful in attenua ting and providing a reliable control of all hemodynamic response changes<br />
accompanied the process of laryngoscopy and intubation. Therefore, all are proved effective<br />
premedication and no one being superior.<br />
Key words: fentanyl, medazolom, lignocaine, propranolol, endotracheal intubation,<br />
hemodynamic response.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﺔﻴﻋﻭﻷﺍﻭ ﺐﻠﻘﻟﺍ ﻝﺎﻌﻓﺃ ﺩﻭﺩﺭ ﺮﻴﺜﻳ ﺎﻤﻫﻼﻛ , ﺮﻳﺪﺨﺘﻟﺍ ءﺎﻨﺛﺃ ﻱﻭﺰﻏ ﺰﻔﺤﻣ ﺖﻴﺒﻨﺘﻟﺍ ﻚﻟﺬﻛﻭ ﺔﻴﺋﺍﻮﻬﻟﺍ ﺔﺒﺼﻘﻟﺍﻭ ﺓﺮﺠﻨﺤﻟﺍ ﺮﻴﻈﻨﺗ ﺮﺒﺘﻌﻳ<br />
ﻭﺃ ﺪﺤﺗ ﻥﺃ ﺎﻬﻧﺎﺷ ﻦﻣ ﺔﻔﻠﺘﺨﻣ ﺐﻴﻟﺎﺳﺃ ﺖﻣﺪﺨﺘﺳﺍ ﺪﻗﻭ , ﻢﻈﻨﻟﺍ ﻞﻠﺧﻭ ﺐﻠﻘﻟﺍ ﺕﺎﺑﺮﺿ ﻡﺎﻈﺘﻧﺍ ﻡﺪﻋﻭ ﻡﺪﻟﺍ ﻂﻐﺿ ﻉﺎﻔﺗﺭﺍ ﻞﻤﺸﺗ ﻲﺘﻟﺍ ﺔﻳﻮﻣﺪﻟﺍ<br />
ﺩﺍﻮﻣ ﺎﻫﺭﺎﺒﺘﻋﺎﺑ ﻝﻮﻟﻮﻧﺍﺮﺑﻭﺮﺒﻟﺍﻭ ﻦﻴﻛﻮﻨﻜﻴﻟﺍ , ﻡﻻﻭﺯﺍﺪﻴﻤﻟﺍ ﻦﻣ ﻞﻛ ﺮﻴﺛﺄﺗ ﻢﻴﻴﻘﺘﻟ ﺔﺳﺍﺭﺪﻟﺍ ﻩﺬﻫ ﻢﻴﻤﺼﺗ ﻢﺗ ﺪﻘﻟ . ﻞﻴﺒﻘﻟﺍ ﺍﺬﻫ ﻦﻣ<br />
ﻞﻌﻔﻟﺍ ﺩﻭﺩﺭ ﻒﻔﺨﺗ<br />
ﻭﺫ ﻰﺿﺮﻤﻠﻟ ﻲﻣﺎﻏﺮﻟﺍ ﺖﻴﺒﻨﺘﻟﺍ ﺔﻴﻠﻤﻋ ﻝﻼﺧ ﺎﻬﻴﻓ ﺏﻮﻏﺮﻤﻟﺍ ﺮﻴﻏ ﻞﻌﻔﻟﺍ ﺩﻭﺩﺭ ﻒﻴﻔﺨﺘﻟ ﺔﻳﻮﻣﺪﻟﺍ ﺓﺭﻭﺪﻟﺍ ﻲﻓ ﻞﻴﻧﺎﺘﻨﻔﻠﻟ ﺔﻤﻴﻗ ﺕﺍﺫ ﺓﺪﻋﺎﺴﻣ<br />
ﻭﺃ ﻝﻭﻷﺍ ﻝﺎﺣ , ﺮﻳﺪﺨﺘﻟﺍ ءﺎﺒﻃﻷ ﺔﻴﻜﻳﺮﻣﻻﺍ ﺔﻴﻌﻤﺠﻟﺍ ﻢﻈﻧ ﻰﻠﻋ ًﺍﺩﺎﻤﺘﻋﺍ ﺖﻔﻨﺻ ﺔﻟﺎﺣ ﻦﻴﺛﻼﺛﻭ ﻦﻴﻨﺛﻻ ﻲﺋﺍﻮﺸﻌﻟﺍ ﻊﻳﺯﻮﺘﻟﺍ ﻢﺗ . ﻲﻌﻴﺒﻄﻟﺍ ﻂﻐﻀﻟﺍ<br />
8 ) ﻊﻴﻣﺎﺠﻣ ﻊﺑﺭﺃ ﻰﻟﺇ ﻢﻬﻤﻴﺴﻘﺗ ﻢﺗ . ﻲﺳﺎﻴﻘﻟﺍ ﺏﺎﺨﺘﻧﻻﺎﺑ ﻡﺎﻌﻟﺍ ﺮﻳﺪﺨﺘﻟﺍ ﺖﺤﺗ ﻢﻬﻟ ﺔﻴﺣﺍﺮﺟ ﺔﻴﻠﻤﻋ ءﺍﺮﺟﺇ ﺭﺮﻘﺗ ﻦﻳﺬﻟﺍ ﻦﻣ , ﻰﺿﺮﻤﻟﺍ ﻦﻣ ﻲﻧﺎﺜﻟﺍ<br />
ﻦﻣ ﻢﻐﻛ<br />
/ ﻡﺍﺮﻏﻭﺮﻜﻴﻣ 1 ﺕﺎﻋﻮﻤﺠﻣ ﻊﺑﺭﻷﺍ ﻲﻓ ﺾﻳﺮﻣ ﻞﻛ ﻰﻘﻠﺗ . ءﺎﻳﻭ ﻡﻻ ٬ ﻢﻴﻣ ٬ ءﺎﻓ ﻑﺮﺣﻷﺎﺑ<br />
ﻢﻬﻟ ﺰﻣﺭﻭ ٬ ( ﺔﻋﻮﻤﺠﻣ ﻞﻛ ﻲﻓ ﻰﺿﺮﻣ<br />
ﻦﻴﻛﻮﻨﻜﻟ ﻢﻐﻛ/<br />
ﻢﻐﻠﻣ 1.5 ﺎﻳﺪﻳﺭﻭ ﻡﻻﻭﺯﺍﺪﻴﻣ ﻢﻐﻛ / ﻢﻐﻠﻣ 0.2 ﺭﺍﺪﻘﻤﺑ ءﺎﻳ ٬ ﻡﻻ ٬ ﻢﻴﻣ ﺕﺎﻋﻮﻤﺠﻣ ﻲﻓ ﻰﺿﺮﻤﻟﺍ ﺞﻟﺎﻌﺗﻭ . ﺎﻳﺪﻳﺭﻭ ﻞﻴﻧﺎﺘﻨﻔﻟﺍ<br />
ﻪﻌﺒﺘﻳ ﻥﻮﺘﻨﺑﻮﻳﺎﺛ ﻡﻮﻳﺩﻮﺼﻟﺍ ﻦﻣ ﻢﻐﻛ/<br />
ﻢﻐﻠﻣ 2 ﻊﻣ ﺮﻳﺪﺨﺘﻟﺍ ءﺍﺮﻘﺘﺳﺍ ﺎﻫﺪﻌﺑ ﻢﺗ . ﻲﻟﺍﻮﺘﻟﺍ<br />
ﻰﻠﻋ ﺎﻳﺪﻳﺭﻭ ﻝﻮﻟﻮﻧﺍﺮﺑﻭﺮﺑ ﻢﻐﻛ / ﻢﻐﻠﻣ 0.01 ﺎﻳﺪﻳﺭﻭ<br />
ﻡﺪﻟﺍ ﻂﻐﺿﻭ ﺐﻠﻘﻟﺍ ﺕﺎﻀﺒﻧ ﻝﺪﻌﻣ ﺱﺎﻴﻗ ﻢﺗﻭ ٬ ﺮﻳﺪﺨﺘﻟﺍ ﺾﻳﺮﺤﺗ ﻦﻣ ﺔﻘﻴﻗﺩ 2 ﺪﻌﺑ ﻲﻣﺎﻏﺮﻟﺍ ﺖﻴﺒﻨﺘﻟﺍ ﺬﻔﻧ.<br />
ﻦﻴﻟﻮﻛ ﻞﻴﻨﺴﻜﺴﻟﺍ ﻦﻣ ﻢﻐﻛ/<br />
ﻢﻐﻠﻣ1.5<br />
ﻖﺋﺎﻗﺩ 4٬6٬8 ٬ 2 ﺪﻌﺑ ﻢﺛ ﺚﺤﻟﺍ ﺪﻨﻋ ﻚﻟﺫﻭ ٬ ءﺍﺮﻘﺘﺳﻻﺍ ﻞﺒﻗ ﺞﺘﻨﻤﻟﺍ<br />
ﻂﻐﺿ ﻝﺪﻌﻣﻭ ﻲﻧﺎﻳﺮﺸﻟﺍ ﻂﻐﻀﻟﺍ ٬ ﻲﻃﺎﺴﺒﻧﻻﺍ ﻡﺪﻟﺍ ﻂﻐﺿ ٬ ﻲﺿﺎﺒﻘﻧﻻﺍ<br />
ءﺎﻨﺛﺃ ﺔﻌﺑﺭﻷﺍ ﺕﺎﺌﻔﻟﺍ ﻊﻴﻤﺟ ﻲﻓ ﺔﻳﻮﻣﺪﻟﺍ ﺓﺭﻭﺪﻟﺍ ﻭﺃ ﺐﻠﻘﻟﺍ ﺩﻭﺩﺭ ﺓﺭﺎﺛﺇ ﺔﺑﺎﺠﺘﺳﺍ ﻲﻓ ﺮﻴﺒﻛ ﻑﻼﺘﺧﺍ ﺩﻮﺟﻭ ﻡﺪﻋ ﺞﺋﺎﺘﻨﻟﺍ ﺖﺤﺿﻭﺃ ٬ ﺖﻴﺒﻨﺘﻟﺍ ﺪﻌﺑ<br />
ﻦﻴﻛﻮﻨﻜﻟﺍ ٬ ﻡﻻﻭﺯﺍﺪﻴﻤﻟﺍ ٬ ﻞﻴﻧﺎﺘﻨﻔﻟﺍ ) ﺔﻌﺑﺭﻷﺍ ﺔﻳﻭﺩﻷﺍ ﻞﺒﻗ ﻦﻣ ﺔﻟﺎﻌﻔﻟﺍ ﻰﻧﺩﻷﺍ ﺪﺤﻟﺍ ﺔﻋﺮﺟ ﻥﺇ ﻦﻴﺒﺗ ﻡﺎﺘﺨﻟﺍ ﻲﻓ . ﺔﻴﺋﺍﻮﻬﻟﺍ ﺔﺒﺼﻘﻟﺍ ﺖﻴﺒﻨﺗ<br />
ﻞﻜﻓ ﺍﺬﻟ ﺎﻬﺑ ﻕﻮﺛﻮﻣ ﺔﺒﻗﺍﺮﻣ ﺮﻴﻓﻮﺗﻭ ﺖﻴﺒﻨﺘﻟﺍﻭ ﺓﺮﺠﻨﺤﻟﺍ ﺮﻴﻈﻨﺗ ﺔﻴﻠﻤﻋ ﺐﺣﺎﺼﺗ ﻲﺘﻟﺍ ﺕﺍﺮﻴﻐﺘﻟﺍ ﻊﻴﻤﺟ ﺓﺪﺣ ﻒﻴﻔﺨﺗ ﻲﻓ ﺔﺤﺟﺎﻧ ( ﻝﻮﻟﻮﻧﺍﺮﺑﻭﺮﺒﻟﺍﻭ<br />
. ﺎﻤﻬﻨﻴﺑ ﺮﺧﻵﺍ ﻦﻣ ﻞﻀﻓﺃ ﻮﻫ ﻦﻣ ﻚﻟﺎﻨﻫ ﺲﻴﻟﻭ ﺔﻠﻀﻔﻣ ﺮﺒﺘﻌﺗ ﺪﻋﺎﺴﻤﻟﺍ ﺩﺍﻮﻤﻟﺍ ﻩﺬﻫ ﻦﻣ<br />
Introduction<br />
Laryngoscopy and intubation are<br />
mandatory for most patients undergoing<br />
surgery under general anesthesia, often<br />
accompanied by a hemodynamic pressor<br />
response (1,2,3) . The rise in pulse rate and blood<br />
pressure is usually transient, variable and<br />
unpredictable; these changes are usually<br />
1Corresponding author E- mail : may_sabbagh @ yahoo.com<br />
Received : 13/4/2010<br />
Accepted : 8/6/2010<br />
24<br />
tolerated by healthy individuals, however, they<br />
may be deleterious in patients with<br />
hypertension, coronary artery diseases or<br />
intracranial hypertension, culminating<br />
perioperative myocardial ischemia, cardiac<br />
arrhythmias, acute heart failure and<br />
cerberovascular accident (4) .
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
Various drugs including calcium channel<br />
(5) (6)<br />
blockers , vasodilators , β-adrenergic<br />
blockers (7,8) , topical and intravenous<br />
(9,10) (11,12)<br />
lignocaine , opioids and deep<br />
inhalational anesthesia (13,14) have been used in<br />
an attempt to attenuate or prevent pressor<br />
responses that accompanied endotracheal<br />
intubation, but non have been satisfactory.<br />
Fentanyl, a synthetic opioid, is one of the<br />
potent analgesics, when used before induction<br />
helps to attenuate hemodynamic response to<br />
(1)<br />
intubation . Medazolam, a short acting<br />
benzodiazepine, most commonly used for its<br />
anxiolytic, muscle relaxant and sedative<br />
properties (15,16) , has slow onset of action with<br />
more gradual effects on circulation and greater<br />
degree of antegrade amnesia than thiopental;<br />
so it may offer an advantage in situation where<br />
hemodynamic stability is crucial (17,18) . Recent<br />
studies suggested that propranolol and osmolol<br />
can also provide consistent and reliable<br />
protection against the increase in both heart<br />
rate and systolic blood pressure that<br />
accompany intubation, and may reduce the<br />
risk of adverse cardiac events in patient<br />
(8,19)<br />
undergoing major surgical operation .<br />
Lignocaine hydrochloride, an amine<br />
ethylamide local anesthetic and class I B-<br />
antidysrrhythmic drug is also acceptable for<br />
attenuation of cardiovascular response to<br />
intubation, and can also diminish cough<br />
reflexes, dysrhythmias and increase in<br />
intracranial pressure (4) . The present study was<br />
designed to evaluate the effects of medazolam,<br />
lignocaine and propranolol, as adjuvants to<br />
fentanyl, on the hemodynamic pressor<br />
response during endotracheal intubation in<br />
normotensive patients.<br />
Patients and Methods<br />
The present study was conducted at the<br />
Neurosurgery Hospital in Baghdad in 2007<br />
and involved thirty two ASA physical status I<br />
or II patients, with age range of 18-45 years,<br />
scheduled for elective surgery, requiring<br />
general anesthesia with endotracheal<br />
intubation. Patients with abnormal<br />
electrocardiogram, significant bronchospastic,<br />
neurologic or cardiovascular diseases,<br />
including those receiving medication known to<br />
affect blood pressure and heart rate were<br />
excluded. On arrival to the operating room,<br />
electrocardiograph monitoring, pulseoximetry<br />
and noninvasive arterial blood pressure<br />
monitoring were applied, and baseline values<br />
of heart rate (HR), systolic blood pressure<br />
(SBP), diastolic blood pressure (DBP), mean<br />
arterial pressure (MAP), and the rate pressure<br />
product (RPP) were obtained. Patients were<br />
randomly allocated into four groups (each<br />
25<br />
include 8 patients) treated as follow: group F<br />
considered as control received only 1µg/kg i.v<br />
fentanyl, group M; patients in this group<br />
received 1µg/kg i.v fentanyl plus 0.2mg/kg i.v<br />
medazolam, group L; received both 1µg/kg i.v<br />
fentanyl and 1.5mg/kg i.v lignocaine, while<br />
group P received 1µg/kg i.v fentanyl together<br />
with 0.01mg/kg i.v propranolol. After 2<br />
minutes of administrating pre-medications,<br />
induction of anesthesia was achieved with<br />
2mg/kg thiopental and 1.5 mg/kg<br />
succinylcholin (all steps and doses were<br />
utilized according to the guidelines adopted in<br />
the neurosurgery hospital, Baghdad). After<br />
loss of eyelash reflex, the lungs were manually<br />
ventilated with oxygen. Direct laryngoscopy<br />
was performed at 2 minutes and trachea was<br />
intubated with proper sized disposable cuffed<br />
tube and fixed after confirmation of proper<br />
position. Following intubation, anesthesia was<br />
maintained with O2, 1% halothane and<br />
pancuronium according to the requirements of<br />
surgery. The follow up of targeted parameters<br />
was started after administration of preanesthetic<br />
medications up to 8 minutes later<br />
on. HR, SBP, DBP and MAP were recorded<br />
before and after induction, then after<br />
intubation every 2 minutes for 8 minutes<br />
interval. The rate pressure product (RPP) was<br />
calculated by multiplying SBP by HR (20) . The<br />
data were statistically evaluated utilizing<br />
paired Student's t-test to compare pre- and<br />
post-treatment values. Intergroup comparison<br />
was performed using unpaired t-test and<br />
ANOVA. Results were considered<br />
significantly different at P
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
intubation. Compared to base line values, both<br />
HR and RPP showed gradual but non<br />
significant increase at 2, 4, 6 and 8 minutes<br />
Table 1: Demographic data of patie nts included in the prese nt study<br />
26<br />
after intubation and reach optimal value at 6-8<br />
minutes of intubation in all groups.<br />
Groups Sex Age (year) Weight (kg) Hb (%) HCT (%)<br />
Gr F 6 M 25.8 ± 8.2 64.0 ± 11.6 13.0 ± 1.7 41.1 ± 5.1<br />
(n=8) 2 F NS<br />
NS<br />
NS<br />
NS<br />
Gr M 5 M 25.6 ± 7.4 64.6 ± 13.9 12.1 ± 1.5 37.0 ± 4.7<br />
(n=8) 3 F NS<br />
NS<br />
NS<br />
NS<br />
Gr L 6 M 27.6 ± 9.0 64.1 ± 18.2 13.1 ± 1.1 38.0 ± 4.2<br />
(n=8) 2 F NS<br />
NS<br />
NS<br />
NS<br />
Gr P 6 M 28.0 ± 9.4 61.9 ± 13.6 13.1 ± 1.5 39.4 ± 5.2<br />
(n=8) 2 F NS<br />
NS<br />
NS<br />
NS<br />
F: Fentanyl group; M: Medazolam group; L: Lignocaine group; P: Propranolol group; Data are<br />
presented as mean ± SD; n=number of patients. NS: non significant<br />
Table 2: Effects of Fentanyl or its combination with Medazolam, Lignocaine or Propranolol on<br />
systolic blood pressure during intubation in surgery<br />
Stages<br />
Systolic Blood Pressure (mmHg)<br />
Groups<br />
F M L P<br />
Pre-induction<br />
(base line)<br />
129.8 ± 22.4 a 135.5 ± 15.1 a,b 125.5 ± 21.5 a 137.4 ± 10.5 b<br />
Induction 121.3 ± 21.2 a,b 129.6 ± 15.7 a,b 126.3 ± 20.8 a 134.6 ± 10.2 b<br />
2 min 126.1 ± 20.7<br />
Postintubation<br />
a 140.9 ± 20.1 a 132.0 ± 20.0 a 139.4 ± 10.7 a<br />
4 min 117.3 ± 11.1 a 127.8 ± 16.3 a,b 127.5 ± 21.2 a,b 134.3 ± 13.1 b<br />
6 min 116.4 ± 8.4 a 124.4 ± 23.1 a 130.9 ± 26.0 a 132.0 ± 33.8 a<br />
8 min 110.8 ± 26.3 a 113.8 ± 24.6 a 117.9 ± 21.9 a 128.6 ± 23.2 b<br />
Data are presented as mean ± SD; number of patients was 8 in each group; no significant difference<br />
existing with respect to induction value; non-identical superscripts (a,b) within the same time represent<br />
significant difference (P
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
Table 4: Effects of Fentanyl or its combination with Medazolam, Lignocaine or Propranolol on<br />
mean arterial pressure during intubation in surgery<br />
Stages<br />
Mean Arterial Pressure (mmHg)<br />
Groups<br />
F M L P<br />
Pre -induction<br />
(base line)<br />
93.6 ± 12.7 a 97.9 ± 12.9 a 91.8 ± 12.2 a 98.5 ± 8.5 b<br />
Induction 90.0 ± 13.3 a 96.0 ± 17.2 a 93.1 ± 12.9 b 96.4 ± 18.3 c<br />
2 min 94.5 ± 22.4<br />
Postintubation<br />
a 101.3 ± 30.3 a 95.4 ± 10.6 b 100.1 ± 8.6 b<br />
4 min 89.6 ± 15.7 a 96.6 ± 13.7 a 91.9 ± 13.4 b 98.3 ± 8.9 b<br />
6 min 88.9 ± 8.0 a 93.8 ± 14.2 a 99.4 ± 19.2 a 95.8 ± 21.9 a<br />
8 min 83.8 ± 13.9 a 82.5 ± 14.3* a 82.6 ± 16.5 a 94.4 ± 25.4 a<br />
Data are presented as mean ± SD; number of patients was 8 in each group; *P
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
Discussion<br />
Laryngoscopy and tracheal intubation<br />
produced stressful hemodynamic changes in<br />
the form of hypertension and tachycardia,<br />
attributed to increase in the circulating levels<br />
of catecholamines<br />
(21,22) . Control of such<br />
hemodynamic changes are very important to<br />
prevent detrimental effects, and the need for<br />
safe and effective therapeutic agents that may<br />
attenuate, blunt, suppress or abolish such<br />
changes became an important intervention<br />
during surgical procedures under general<br />
anesthesia. The results obtained from the<br />
present study revealed that all studied patients<br />
groups showed quantitatively and qualitatively<br />
similar hemodynamic pressor response at<br />
induction, intubation and post-intubation; the<br />
differences, if present, failed to reach<br />
statistically significant values. In the present<br />
study, failure to predict superiority for each<br />
pattern of drug intervention may be attributed<br />
to the limited number of patients in each<br />
group, and increase the number of patients<br />
may lead to more predictable values.<br />
However, pre-operative use of minimum<br />
effective doses of pre-anesthetic medications<br />
(1μg/kg fentanyl, 0.2mg/kg medazolam,<br />
1.5mg/kg lignocaine and 0.01mg/kg<br />
propranolol) in the present study was found to<br />
be effective in restricting the non-significant<br />
increase in SBP, DBP and MAP values during<br />
short period of time (up to 2 minutes postintubation),<br />
then each parameter start to<br />
decrease gradually (but non-significantly) until<br />
8 minutes post- intubation; this means that all<br />
studied medications produce consistent and<br />
reliable protection against the abnormal<br />
increase in hemodynamic pressor response<br />
during laryngoscopy and intubation, similar to<br />
observations reported by other investigators<br />
(4,8,19,23) . Additionally in the present study, a<br />
non-significant increase in mean pulse rate and<br />
rate pressure product (good indicator for<br />
oxygen consumption) was reported in all<br />
groups of operated patients, starting from<br />
intubation and reach optimal values after 6-8<br />
minutes post-intubation; this could be<br />
explained by the fact that surgical intervention<br />
usually starts after 6-8 minutes postintubation,<br />
which is by itself a stressful<br />
procedure, predominantly suppresses the<br />
pressor response more effectively than<br />
tachycardia as a response (24) . Light anesthesia<br />
(fewer drugs by the intravenous route or via<br />
inhalational means) is claimed to be the major<br />
factor responsible for pre-operative awareness<br />
and hemodynamic instability (17) ; to overcome<br />
this problem, fentanyl and/or medazolam are<br />
administered for the purpose of analgesia,<br />
sedation and anxiolysis (16) . Many evidence<br />
28<br />
indicated that each of them, when used alone<br />
or in combination, enables reduction of the<br />
thiopental dose required to produce induction,<br />
and consequently limit potential side effects<br />
and help in attenuating the hemodynamic<br />
response to laryngoscopy and intubation<br />
(16,25,26)<br />
. Despite the potential advantages of the<br />
drug combination, reluctance to incorporate<br />
medazolam during light anesthesia persists<br />
due to concern regarding the potential for<br />
prolonged recovery (27,28) . However, a small<br />
pre-induction bolus dose of medazolam<br />
utilized in the present study did not prolong<br />
both recovery and discharge time from the day<br />
care unit following general anesthesia; this can<br />
be explained by the fact that the effects of<br />
medazolam on CNS is dose dependent (27) . In<br />
the present study, although fentanyl was<br />
administrated in relatively small doses, it<br />
produces sufficient analgesia for short surgical<br />
procedures, and no one of the operated<br />
patients experienced pain of relatively long<br />
duration or great severity. Although there is a<br />
possibility that administration of narcotic<br />
analgesic like fentanyl may affect<br />
pharmacokinetics of the anesthetic agents<br />
during induction, which is mostly due to<br />
changes in hemodynamic response (29,30,31) , the<br />
patients in F and M groups showed nonsignificant<br />
increase in HR and RPP during<br />
laryngoscopy up to 6 minutes post-intubation;<br />
this increase seems to be suppressed in<br />
medazolam-treated group compared to<br />
fentanyl-treated group. This indicates that<br />
administration of medazolam before induction<br />
lead to hemodynamic stability most probably<br />
by mutual potentiation (32) .Many studies have<br />
reviewed the effect of lignocaine to blunt the<br />
hemodynamic response after endotracheal<br />
intubation (4) . It has been reported that the<br />
strength and timing o f lignocaine<br />
administration are equally important to<br />
prevent hemodynamic changes (33) , however,<br />
irrespective of the dose and time of<br />
administration of lignocaine, there are still<br />
significant increase in hemodynamic<br />
parameters after intubation (34) . Kindler et al<br />
and Durrani et al reported that i.v.<br />
administration of 1.5mg/kg lignocaine did not<br />
prevent the increase in hemodynamic response<br />
associated with laryngoscopy and intubation<br />
(35,36)<br />
. Meanwhile, other investigators reported<br />
that 1.5mg/kg lignocaine effectively blocked<br />
the increase in SBP, DBP and HR after<br />
intubation<br />
(4,9) . In the present study, i.v<br />
administration of 1.5mg/kg lignocaine, 2<br />
minutes before intubation provide reliable<br />
protection against the rise in hemodynamic<br />
response that associated with intubation<br />
process; this result was in accordance with
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
many previously reported data, but not<br />
consistent with others (4,9) . Such effect may be<br />
attributed to rapid equilibration of lignocaine<br />
between blood and brain with production of<br />
sedative effect when administrated in<br />
appropriate dose (37) . Blocking and blunting<br />
adrenergic responses of tracheal intubation is<br />
the key pathophysiological step connecting βblockers.<br />
Most of the studies concerned with<br />
evaluating the benefit of β-blockade on<br />
mortality and myocardial ischemia after<br />
tracheal intubation are based on using ultrashort<br />
acting selective β-blockers (38,39) , while<br />
very limited reports were available about using<br />
propranolol in this respect (19) . In the present<br />
study, 0.01mg/kg i.v propranolol was used,<br />
and no significant differences were reported in<br />
HR and RPP between patients groups. Even a<br />
slight rise in HR and RPP that occurs at 6<br />
minutes postintubation (a time of surgical<br />
intervention) was non significantly slowered<br />
in the β-blockade group in compare to other<br />
groups. These results are in accordance with<br />
those reported by Hussain et al and Yutaka et<br />
al (7,39) . The results of the present study shed a<br />
light on the possibility of using minimum<br />
doses of thiopental sodium for induction and<br />
maintenance of light anesthesia, for the aim of<br />
decreasing the time to discharge the patient<br />
from the recovery room and the day care unit;<br />
this situation seems to be compatible with the<br />
condition of shortage in medications required<br />
for anesthesia. In conclusion, minimum<br />
effective doses of pre-anesthetic medications<br />
(fentanyl, medazolam, lignocaine and<br />
propranolol) can maintain hemodynamic<br />
stability during laryngoscopy and intubation.<br />
References<br />
1. Channaiah VB, Chary K, Vik JL, Wang<br />
Y. Low-dose fentanyl: hemodynamic<br />
response to endotracheal intubation in<br />
normotensive patients. Arch Med Sci<br />
2008; 4:293- 299.<br />
2. Boralessa H, Senior DF. Cardiovascular<br />
response to intubation. Anaesthesia 1983;<br />
38: 623-627.<br />
3. King BD, Harris LC, Grieleuestein FE,<br />
Elder JD. Reflex circulating response to<br />
direct laryngoscopy and tracheal<br />
intubation performed during general<br />
anesthesia. Anesthesiology 1951; 12:556.<br />
4. Malde AD, Sarode V. Attenuation of the<br />
hemodynamic response to endotracheal<br />
intubation: fentanyl versus lignocaine.<br />
The Internet J Anesthesiol 2007; 12:1.<br />
5. Govindaiah MH, Suryanaayana VG. Can<br />
calcium and sodium channel blockers<br />
attenuate hemodynamic response to<br />
29<br />
endotracheal intubation? Eur J Gen Med<br />
2008; 5(4):198-207.<br />
6. Kamra S, Wig J, Sapru RP. Topical<br />
nitroglycerin. A safeguard against pressor<br />
responses to endotracheal intubation.<br />
Anesthesia 1986; 41:1087-1091.<br />
7. Hussain AM, Sultan ST. Efficacy of<br />
fentanyl and esmolol in the prevention of<br />
hemodynamic response to laryngoscopy<br />
and endotracheal intubation. J Coll<br />
Physicians Surg Pak 2005<br />
; 15(8):454-457.<br />
8. Ogurlu UB, Erdal MC, Aydin ON. Effects<br />
of Esmolol, Lidocaine and Fentanyl on<br />
haemodynamic responses to endotracheal<br />
intubation: A comparative study. Clin<br />
Drug Invest 2007; 27:269-277.<br />
9. Kim WY, Lee YL, Ok SJ, Chang MS.<br />
Lidocaine does not prevent bi-spectral<br />
index increases in response to<br />
endotracheal intubation. Anesthesia Analg<br />
2006; 102: 156-159.<br />
10. Hamill JF, Bedford RF, Weaver DC,<br />
Colohn AR. Lidocaine before<br />
endotracheal intubation: intravenous or<br />
laryngotracheal? Anesthesiology 1981;<br />
55:578-581.<br />
11. Dahlgren N, Messeter K. Treatment of<br />
stress response to laryngoscopy and<br />
intubation with fentanyl. Anesthesia 2007;<br />
36:1022-1026.<br />
12. Adachi YU, Satomoto M, Higuchi H.<br />
Fentanyl attenuates the hemodynamic<br />
response to endotracheal intubation more<br />
than the response to laryngoscopy.<br />
Anesthesia Analg 2002; 95:233-237.<br />
13. Sklar BZ, Lurie S, Ezri T, Krichelli DL.<br />
Lidocaine inhalation attenuates the<br />
circulatory response to laryngoscopy and<br />
endotracheal intubation. J Clin Anesthesia<br />
1992; 4(5):382-385.<br />
14. Kautto UN, Saarnivaaral A. Attenuation<br />
of the cardiovascular intubation with NO2,<br />
Halothane or enflurane. Acta Anaesthesiol<br />
Scand 1983; 27:289-293.<br />
15. Kim HK, Chung YJ, Lee MS.<br />
Comparison of medazolam and thiopental<br />
as an induction agent. Korean J<br />
Anaesthesiol 1991; 24(4):826-832.<br />
16. Lohakare R, Sangawar AV, Ghosh AA.<br />
Influence of intravenous fentanyl and/or<br />
Medazolam on induction of anesthesia<br />
with thiopentone. J Anaesth Clin<br />
Pharmacol 2004; 20(3): 273-278.<br />
17. Khan AM, Akan F. Midazolam and<br />
thiopentone co-induction: looking for<br />
important in quality of anaesthesia. JPMA<br />
2003; 53:542-547.
Iraqi J Pharm Sci, Vol.19(2) 2010 Preanesthetic medications and hemodynamic changes<br />
18. Reves JG, Fragen R J, Vinik HR, et al.<br />
Midazolam: pharmacology and uses.<br />
Anesthesiology1985 ; 62:310-324.<br />
19. Chae DH, Park KJ. Effect of verapamil<br />
and propranolol on heaemodynamic<br />
response to laryngoscopy and tracheal<br />
intubation in hypertension patients.<br />
Korean J Anesthesiol 1990; 23(3) :366-<br />
372.<br />
20. Rathore A, Gupta HK, Tanwar GL.<br />
Attenuation of the pressure response to<br />
laryngoscopy and endotracheal intubation<br />
with different doses of osmolol. Indian J<br />
Anaesth 2002; 46(6):449-452.<br />
21. Asad N, Ai K, Qayyum A. Effect of<br />
Nalbuphin and midazalam on<br />
hemodynamic response to intubation.<br />
Canadian J Anesthesia 2006; 53: 26192.<br />
22. Kyung Y, Un LJ, Hak SK. Hemodynamic<br />
and Catecholamine response to<br />
Laryngoscopy and Tracheal intubation in<br />
patient with complete spinal cord injuries.<br />
Anesthesiology 2001; 95(3):647-65.<br />
23. Im ES, Jeon DG, Shine HC. Effect of<br />
fentanyl, medazolam and fentanyl-<br />
medazolam on the cardiovascular system<br />
and blood glucose during general<br />
anesthetic. J Korean Soc Anesthesiol<br />
1994; 27(9):1083-1091.<br />
24. Constant I., naghe M.C, Boudt L,B.<br />
Reflex papillary dilatation in response to<br />
skin incision and alfantanil in children<br />
anaesthetized with sevoflurane : a more<br />
sensitive measure of noxious stimulation<br />
than the commonly used variables. British<br />
Journal of anesthesia 2006;96(5);614-<br />
619.<br />
25. Dundee JW, Halliday NJ. Pretreatment<br />
with opiods. The effect of thiopentone<br />
induction requirements and overset of<br />
action of medazolam. Anesthesia 1986;<br />
41:159-161.<br />
26. Vinik HR. Anesthetic interactions. Eur J<br />
Anesthesiol 1995; 12:3-4.<br />
27. Miller DR, Blew PG, Martineau RJ.<br />
Midazolam and awareness with recall<br />
during total intravenous anesthesia. Can J<br />
Anaesth 1996; 439(9):946-953.<br />
28. Delucia JA, White PF. Effect of<br />
medazolam on induction and recovery<br />
characteristics of propranolol. Anesth<br />
Analg 1992; 74:563.<br />
29. Adachi YU, Watanabe K, Higuchi H,<br />
Satoh T. The determinants of propofol<br />
induction of anesthesia dose. Anesth<br />
Analg 2001; 92:656-661.<br />
30<br />
30. Kazama T, Ikeda K, Morita K. et al.<br />
Relation between initial bloods<br />
distribution volume and propofol<br />
induction dose requirement.<br />
Anesthesiology 2001; 94:205-210.<br />
31. Cocksholl ID, Briggs LP, Douglas EJ,<br />
White M. Pharmacokinetics of propofol in<br />
female patients: studies using single bolus<br />
injections. Br J Anaesth 1987; 59:1103-<br />
1110.<br />
32. Shlomo B, Abdelkhalim H. Midazolam<br />
act synergistically with fentanyl for<br />
induction of anaesthesia. Br J Anaesth<br />
1990; 64:45-47.<br />
33. Wang YM, Chung KC, Huang YM.<br />
Lignocaine the optimal timing of<br />
intravenous administration in attenuation<br />
of increase of intraocular pressure during<br />
tracheal intubation. Acta Anaesthesiol Sin<br />
2003; 41(2):71-75.<br />
34. Miller CD, Warren SJ. I.V Lignocaine<br />
fails to attenuate the cardiovascular<br />
response to laryngoscopy and tracheal<br />
intubation. Br J Anaesth 1990; 65(2):216-<br />
219.<br />
35. Kindler CH, Schumacher PG, Schneider<br />
MC. Effects of intravenous lidocaine<br />
and/or esmolol on hemodynamic response<br />
to laryngoscopy and intubation; a doubleblind,<br />
controlled clinical trial. J Clin<br />
Anesth 1996; 8:491-496.<br />
36. Durani M, Barwise JA, Johnson RC, et al.<br />
Intravenous chloroprocaine attenuates<br />
hemodynamic changes associated with<br />
direct laryngoscopy & tracheal intubation.<br />
Anesth Analg 2000; 90:1208-1212.<br />
37. Nishino T, Hiraga k, Sugimori K. Effects<br />
of i.v. Lignocaine on airway reflexes<br />
elicited by irritation of the tracheal<br />
mucosa in humans anaesthetized with<br />
enflurane. Br J Anaesth 1990; 64:682-<br />
687.<br />
38. Sugiura S, Seki S, Hidaka K. The<br />
hemodynamic effects of landiolol, an<br />
ultra-short-acting beta1-selective blocker,<br />
on endotracheal intubation in patients<br />
with and without hypertension. Med Ac<br />
Jp 2007; 22:123-126.<br />
39. Yutaka O, Nishikawa K, Hase I. The<br />
short-acting β1-adrenoceotor antagonists<br />
esmolol and landiolol suppress the<br />
bispectral index response to tracheal<br />
intubation during sevoflurane anesthesia.<br />
Anesth Analg 2005; 100:733-737.
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
Validity of Generalized Standard Addition Method for a Mixture of<br />
Amino Acid Analysis<br />
Azhar M. Jasim* ,1<br />
*Departement of Pharmaceutical Chemistry,College of Pharmacy,University of Baghdad,Baghdad, Iraq.<br />
Abstract<br />
A Modified version of the Generlized standard addition method ( GSAM) was developed. This<br />
modified version was used for the quantitative determination of arginine (Arg) and glycine ( Gly) in<br />
arginine acetyl salicylate – glycine complex . According to this method two linear equations were<br />
solved to obtain the amounts of (Arg) and (Gly). The first equation was obtained by spectrophotometic<br />
measurement of the total absorbance of (Arg) and (Gly) colored complex with ninhydrin . The second<br />
equation was obtained by measuring the total acid consumed by total amino groups of (Arg) and ( Gly).<br />
The titration was carried out in non- aqueous media using perchloric acid in glacial acetic acid as a<br />
titrant. The developed method is accurate, precise, and free from interferences and may provide a<br />
useful approach to calibrate in the direct analysis of solid sample and it is suitable method to be used as<br />
a quality control procedure.<br />
Key words: GSAM , amino acid analysis .<br />
31<br />
ةصلاخلا<br />
ٌخٗمم ىو ضٍموموخ ُ م خوخ تطمضو طٌٕخماخم أ هٕمله هممشب GSAM تمٌط وخ ت م ط ةوخ تاطمساخ تمة طض اممىي ط ٗم ح نطملاخ ًمح<br />
تةوم ط ووخ ومٌا ًمح ُ مخ ل ُ خواطم ٌ امىي نٗمل ىوٖ ُ مل لال – ج ى ل وموط هوم طوخ أ ُوم ّويسأ مةوم ٍووخ ـم لطح ضما تىلخموموخ تم ّ ٌاخ<br />
ٌطممض يخماخمم خب أ ض طممٌ مم ٌ ضمما مملخوخ تممة طضٖ امموٖاخ تممواط ٍوخ اممىي نٗممل ىو ُ سمممّّٕوخ نٗممى ٌ يخماخمم خب أ ت ِٗوممىوخ<br />
ٌطمض تاطمسخب ممة ٍوخ ُمٌ م م أ كى مل وط ه طخ م اخ نيمي مم ب كموذٖ٬<br />
تم ِط وخ تمواط ٍوخ امىي نٗمل ىو ملٌ نٗمى ٍل كم سٗىلط بوخ<br />
تممة ط ب ٓمخ ٍ ل ط مممة حٖ ط م اخ ٗ مو وخ ـ ممٍ وخ ت م خٗ ب س ط م خٍ وخ م مم أ كى مل وط ه طخ م اخ ( الا سطم ياخمم<br />
٥٫٠أك<br />
أٖ سٗىلٖسمم ٕوخ<br />
ُمٌ تم وطلٖ تة قا طخّوخٖ ت ط تة ط وخ ْ ٔ َٗل اىي جوا طٕ وخ هصٗخوخ ًح ضخوخ طخّوخ . ضيطيساخٖأ طشطبٍوخ لخوخ أ لخوخ<br />
. ةمة ٌ ةيٕيا جطخ ح ٖ ت ٖط ٍ موخ ثلالخمخوخ<br />
Introduction<br />
Numerous methods for the analysis of<br />
acetylsalicylic acid could be found in the<br />
literature (1-10) . However, these methods are not<br />
suitable for the analysis of acetylsalicylic acid<br />
mixture with arginine and glycine. Argnine<br />
and glycine interfere with direct titrimetric<br />
determination of acetylsalicylic acid therefore,<br />
it was intended to separate acetylsalicylic acid<br />
quantitatively from its salts before it is<br />
determined by titrimetric method. It was<br />
intended to use spectrophotometry or titrimetry<br />
for determination of arginine and glycine .<br />
These two methods are simple and therefore<br />
the most widely used in routine drug and many<br />
compounds analysis as could be seen from the<br />
latest version of British , Swiss and United<br />
State Pharmacopeias. However, direct<br />
application of these methods for determination<br />
of arginine and glycine in a mixture containing<br />
both of them is not possible because they<br />
interfere with each other. As a remedy for this<br />
situation it was decided to use the GSAM<br />
which has wide applications (11-17) . It is based<br />
on the principle of varying both of the sample<br />
molar concentration and pH of solution.<br />
Aimed at the validation and standardization of<br />
analytical procedures with direct solid sample<br />
contain two types of amino acids without<br />
1Corresponding author E- mail : azharmjk@yahoo.com<br />
Received : 11/5/2010<br />
Accepted : 27/6/2010<br />
previous isolation. Many methods were used<br />
for the determination of arginine and other<br />
amino acid involved previous isolation like the<br />
classical analytical technique uses automated<br />
amino acid analyzers, However these methods<br />
require expensive dedicated equipment duo to<br />
the post- column derivatization of the amino<br />
acids , long assay times and large samples<br />
volumes ( 18 ) .Also the studies of PITC as the<br />
derivatizing agent, following the Pico-Tag<br />
method used for the determination of arginine<br />
& other amino acid , those studies require the<br />
establishment of standard–added curve (by<br />
application of the standard addition method )to<br />
avoid proportional errors (19) . The non-aqueous<br />
titration was used for detection of free amino<br />
group in amino acid (20) .This method used for<br />
the quantitative determination of many<br />
chemical compounds and drugs in<br />
pharmaceutical forms, providing precise and<br />
accurate results, which could be verified by<br />
statistical methods (21-23) . A spectrophotometric<br />
method are widely used for determination of<br />
amino acid based on the reaction with<br />
coloring agent at different pH forming colored<br />
complex that measured at specific<br />
wavelength (24-27) .
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
Experimental<br />
Material and Methods<br />
Glacial acetic acid (99.8 %) was obtained<br />
from sigma. Standard perchloric acid (0.1 ml)<br />
in glacial acetic acid was prepared by diluting<br />
(4.25 ml) of perchloric acid (72%) to (500 ml)<br />
with glacial acetic acid containing (10 ml)<br />
acetic anhydride. The prepared solution was<br />
standardized by titration with standard<br />
anhydrous sodium carbonate (0.106 gm) in<br />
glacial acetic acid (50 ml) the titration end<br />
point was determined potentiometrically with<br />
potentiometric titration equipped with glass –<br />
calomel combined electrode. The prepared<br />
standard was used in the titrimetric procedure<br />
for the determination of arginine and glycine<br />
in arginine acetylsalicylate–glycine complex.<br />
Anhydrous sodium carbonate (99.5 %) ,<br />
sodium hydroxide (0.5 M) and hydrochloric<br />
acid (0.5 M) were Aldrich standards . Arginine<br />
(98%, sigma) was standardized to determine<br />
the exact percentage of arginine by titration<br />
with standard hydrochloric acid (0.5 M).<br />
Glycine (99%, fluka) was used after drying in<br />
an oven for 1hr at (105ºC). Acetylsalicylic acid<br />
was purified by recrystilization from ethanol/<br />
water mixture and standardized with standard<br />
(0.5 M) sodium hydroxide (10) . The determined<br />
percentage of acetylsalicylic acid was (99.4<br />
%).The standards arginine , glycine and<br />
acetylsalicylic acid were used to prepare<br />
arginine acetylsalicylate – glycine complex .<br />
The later was used to test the validity of the<br />
proposed method. Sodium acetate buffer (4M,<br />
pH 5.5) was prepared by dissolving sodium<br />
acetate trihydrate NaOAc.3H2O (BDH<br />
“Analar “99.9) in 200 ml of deionized water.<br />
The solution was heated with stirring at (60ºC<br />
) until a clear solution was obtained. Glacial<br />
acetic acid (50 ml) was added and the volume<br />
was completed to (500 ml) with distilled water.<br />
The pH was adjusted by a drop wise addition<br />
of sodium hydroxide (4M) to pH (5.5).<br />
Ninhydrin reagent was prepared as<br />
following : ninhydrine ( I gm) in 2 - methoxy<br />
ethanol (25 ml) and stannous chloride<br />
SnCl2.2H2O (0.07 g) with continuous stirring<br />
until completely dissolved (28) . Sodium acetate<br />
buffer (8.3 ml) was added and the resulting<br />
solution was immediately transferred to dark<br />
glass reservoir bottle and a steam of nitrogen<br />
gas was babbled into the reagent solution for<br />
approximately (20 min). Titration was made<br />
with potentiometric titrator (Metrohm ,<br />
Switzerland ) titro process or equipped<br />
with(Metrohm).Dosimate and glass calomel<br />
combined electrodes. Spectrophotometric<br />
measurement was performed on carry (100<br />
conc) uv- visible spectrophotometer using (1<br />
cm) cells.<br />
32<br />
Determination of acetyl salicylic acid in this<br />
complex<br />
(1gm) of arginine acetylsalicylate–<br />
glycine complex was weighed and dissolved in<br />
(25 ml) of distilled water. The salt was<br />
converted to free acetyl salicylic acid by<br />
acidification with hydrochloric acid (3 M) until<br />
pH (2.7) was reached. The acetyl Salicylic<br />
acid was extracted by ether (3 × 20 ml). The<br />
ether portions were pooled together and ether<br />
was evaporated by a rotary evaporator under<br />
vacuum. The acetyl salicylic acid was<br />
collected to be determined quantitatively by<br />
back titration method or by direct titration<br />
method (5, 10) . The result obtained for acetyl<br />
salicylic acid was corrected for the presence of<br />
salicylic acid as an impurity during liberating<br />
and separating acetyl salicylic acid form the<br />
complex. Salicylic acid was then determined in<br />
the liberated materials (29) .<br />
Determination of arginine and glycine in this<br />
complex<br />
This was achieved by constructing and<br />
solving equations (2) and (4) using ninhydrin<br />
spectrophotometric and titrimetric method in<br />
non – aqueous media respectively.<br />
Spectrophotometric Method<br />
(36 mg) of the complex was precisely<br />
weighed and dissolved in (50 ml) distilled<br />
water to prepare a stock solution. An aliquot (1<br />
ml) of this stock solution was diluted to (25<br />
ml) with distilled water. An aliquot ( 1 ml ) of<br />
this solution was mixed with ( 1ml ) of<br />
ninhydrin reagent in a stoppered test tube ,<br />
shacked and placed in a boiling water bath for<br />
( 15 min ). Ethanol (2.5 ml) and sodium<br />
acetate buffer (2.5 ml) were added to the<br />
mixture which was then cooled below (30 ºC ).<br />
The sample was shacked thoroughly for (30<br />
second) and absorbance was measured at (570<br />
nm) against a reagent blank using (1<br />
cm).Standard curves for arginine and glycine<br />
were constracted using (0.05 = 0.2 mM)<br />
standard solution for each. The values of the<br />
molar absorpitivity coefficient aA and aG in<br />
equation (2) were determined from slops of<br />
these calibration curves.<br />
Titrimetric Method in non – aqueous media<br />
(0.3 gm) of the complex was dissolved in<br />
glacial acetic acid (50 ml) in a dry flask. The<br />
glass electrode of the titroprocessor was<br />
immersed in the solution and the mixture was<br />
titrated against standard perchloric acid<br />
(reagent 1). A plot of pH against the titrant<br />
volume was constructed to obtain the titration<br />
end point.<br />
Stability study<br />
Identical aqueous solution (0.1 g/ 100<br />
ml) of the complex was made. The stability<br />
was determined at different temperatures (25,
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
40, 50, 60 & 70 ºC ) incubated in ovens for<br />
certain intervals. The decomposition of<br />
arginine acetylsalicylate – glycine complex<br />
was indicated by the release of acetylsalicylic<br />
acid , arginine and glycine as appears on TLC<br />
using a solvent system of ( n-propanol : 34%<br />
ammonia 7 :3 ) . The appearance of three<br />
spots on TLC plates for acetyl salicylic acid,<br />
arginine and glycine when the samples were<br />
incubated at (50, 60 & 70 ) ºC . While<br />
incubation at (25 and 40) ºC showed only one<br />
spot for arginine acetylsalicylate – glycine<br />
complex.<br />
Effect of Buffer<br />
Glycine was used as a buffer to stabilize<br />
arginine acetylsalicylat complex. The<br />
concentration of glycine is (0.5 M) in respect<br />
to arginine (1M) to maintain the pH of the<br />
preparation at (4.7).<br />
Result and Discussion<br />
Method of calculation<br />
The GSAM is a traditional vector –<br />
matrix notation to construct and to solve a<br />
system of (n) linear equation in order to<br />
determine the concentration of a mixture of (n)<br />
substances. The mixture of compounds<br />
interferes with each other when measured by<br />
(n) different sensors that belong to any suitable<br />
analytical technique. According to GSAM, the<br />
following system of two linear equations is<br />
required to determine arginine and glycine:<br />
A1 = a11%A+ a12 % G<br />
A2 = a21%A+ a22 % G<br />
……… (1)<br />
…….زز<br />
Where ; A1 and A2 are the total responses of<br />
two analytical sensors ( 1 and 2 ) to the<br />
percentage of arginine ( % A ) and glycine ( %<br />
G ) in the sample the factors a11 – a22 are<br />
absorptivity constant multiplied by the optical<br />
path length ( i.e of the sample ) .Theory of<br />
GSAM equations (1) gives precise result for<br />
%A and %G when the following two<br />
conditions are fulfilled: Firstly there should be<br />
a large difference in magnitude between the<br />
ratio a11/a12 and the ratio a21/a22. Secondly<br />
the precision is measuring A1 and A2 should<br />
be high. These requirements arise from the fact<br />
that the mathematical manipulation magnify<br />
the random error in measurement , so that<br />
large random error is produced in the<br />
calculated concentration of the measured<br />
substances ( i.e %A and %G ) (11,12) .According<br />
to the requirements stated in these two<br />
conditions, it was decided to construct one of<br />
the equations of the system by using<br />
spechtrophotometric technique, while the<br />
second equation is to be constructed using<br />
titrimetic method. Ninhydrin was chosen as a<br />
33<br />
color developing reagent in the<br />
spechtrophtometric procedure, as it is the most<br />
selective among other coloring agent for<br />
spechtrophotometric determination for amino<br />
acids (30-31) .The titrimetric method was used to<br />
obtain the second equation which could not be<br />
performed in aqueous medium because of the<br />
neutral behavior of salts in aqueous medium<br />
that prevent the occurrence of a clear titration<br />
end point . Accordingly, a titrimetric method<br />
in non- aqueous media was adopted using<br />
glacial acetic acid as a solvent and a solution<br />
of perchloric acid in glacial acetic acid as a<br />
titrant. This method was used for quantitative<br />
determination of salt of amines with carboxylic<br />
acids (32-33) .The presence of an acidic salts in<br />
non-aqueous solvent behave as bases and<br />
therefore produce a clear titration end point<br />
when titrated with strong acid (34) .This<br />
experiment of procedure was intended to<br />
produce two linear equations in which the ratio<br />
a11/a12 and a21/a22 are very different in<br />
magnitudes. This was expected from the fact<br />
that arginine and glycine have nearly the same<br />
efficiency to produce colored complexes with<br />
ninhydrin reagent in spectrophotometric<br />
procedure<br />
(31) .While in the titrimetric<br />
procedure arginine molecules have two<br />
titratable amino groups in contrast to the<br />
glycine molecules which have one titratable<br />
group. The first equation which is obtained<br />
from the spectrophotometric procedure was<br />
derived starting from Lambert – beer law as<br />
follows :<br />
… (2)<br />
Equation (2) can be abbreviated as follows:<br />
A = a11 %A + a12 %G ……… (3)<br />
In equation (2) Wasg gm of arginine acetyl<br />
salicylate – glycine complex sample was<br />
dissolved to prepare (vo ml) of stock solution.<br />
This stock solution was then subjected to serial<br />
dilutions (n) with f1,f2…fn dilution factors to<br />
reach the required final concentration.<br />
Mw A and Mw G are the molecular weight of<br />
arginine and glycine respectively. aA and aG<br />
are the molar absorpitivity constants of<br />
arginine and glycine respectively.The use of a<br />
combination of titrimetry and<br />
spechtrophotometry satisfies the requirements<br />
stated in (2). The precision of the<br />
spectrophotometric method is moderate in
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
general.While; the precision of the titrometric<br />
method is very high. Therefore, the use of non-<br />
aqueous titration method improves the overall<br />
precision of the results obtained from such<br />
combination of methods. The second equation,<br />
was obtained from titrimetric procedure as<br />
follows:<br />
Equation (4) can be abbreviated as below:<br />
…(4)<br />
MpVp = a21%A + a22%G …….. (5)<br />
Equation (4) V ml of glacial acetic acid<br />
containing V asg of the analyzed arginine<br />
acetyl Salicylic-glycine complex was titrated<br />
with Mp molar standard of perchloric acid, so<br />
that Vp ml of the standard was required to<br />
reach the end point . Equations (3) and (5) are<br />
mathematically compatible and can be solved<br />
linearly. The ratio a11/a12 and a21/a22 have<br />
large differences in their magnitudes since<br />
a11/a12 ≈ 2 while a 21/a 22 ≈ 1.<br />
Development of Titrimetric Procedure for<br />
Acetyl salicylic acid determination<br />
The complex sample solution must be<br />
acidified to liberate acetyl salicylic acid form<br />
its salt with arginine before extraction by<br />
ether. The optimum pH of the acidified<br />
aqueous solution was determined<br />
experimentally by applying the sample<br />
complex at different pH values of the extracted<br />
sample solution. The result of this study<br />
indicates that pH (2.7) is the optimum pH for<br />
acetyl salicylic acid extraction. At pH higher<br />
than (2.7) low result for acetyl salicylic acid<br />
were obtained due to incomplete liberation<br />
acetyl salicylic acid from its salt with<br />
arginine.The extraction step is vital in the<br />
development procedure because an attempt to<br />
perform direct titration with hydrochloric acid<br />
without performing apparent titration end<br />
point as could be seen from (Figure 1).<br />
Figure 1: Titration curve of arginine<br />
acetylsalicylate -glycine solution with<br />
standard hydrochloric acid (0.5).<br />
34<br />
Analysis of Arginine Acetyl salicylate –<br />
Glycine complex<br />
Quantitative Determination of Acetyl salicylic<br />
acid.<br />
Acetyl salicylic acid was determined<br />
quantitatively by hydrolysis and back titration<br />
method (29) . The result of the two methods was<br />
summarized in table (1).<br />
Table 1: result of acetylsalicylic acid,<br />
arginine and glycine in arginine acetyl<br />
salicylate – glycine complex<br />
Item<br />
Acetylsalicylic<br />
Acid<br />
Arginin<br />
Glycine<br />
Expected<br />
Percentage<br />
W/W<br />
50.0<br />
40.0<br />
10.0<br />
Calculated<br />
Percentage<br />
( w/w ) ± SD<br />
49.6 ± 0.2 *<br />
49.6 ± 0.3 *<br />
40.3 ± 0.6<br />
9.7 ± 0.2<br />
* Hydrolysis and back titration method.<br />
* *Direct titration method.<br />
Quantitative determination of arginine and<br />
glycine in the complex<br />
The quantitative determination of<br />
arginine and glycine in the complex using a<br />
modified version of the GSAM was achieved<br />
by a combination of colorimetric method ( to<br />
obtain the first equation ) and non-aqueous<br />
titration method ( to obtain the second<br />
equation).The colorimetric method using the<br />
colored complex of arginine and glycine with<br />
ninhydrin was applied once at different pH<br />
media and once by using different<br />
wavelengths; the results of these experiments<br />
were summarized in table (2) and (3) and in<br />
figure (2) and (3) respectively.<br />
Table 2: Molar absorptivity constant a* of<br />
arginine and glycine colored complex with<br />
ninhydrin at different pH values measured<br />
at 570nm<br />
pH of aA<br />
ninhydrin<br />
solution<br />
5.5 0.83<br />
7.0 1.07<br />
9.0 1.73<br />
aG<br />
0.62<br />
0.78<br />
0.97<br />
*Represent the Molar absorptivity<br />
multiplied by the cell width<br />
aA / aG<br />
1.34<br />
1.37<br />
1.41<br />
C.V<br />
0.4 %<br />
0.6 %<br />
1.5 %<br />
2.0 %
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
Table 3: Molar absorptivity constant a*<br />
of arginine and glycine colored complex<br />
with ninhydrin at different wavelengths.<br />
pH of<br />
ninhydrin<br />
solution<br />
Wavelength<br />
hs nm<br />
244<br />
aA<br />
1.19<br />
aG<br />
0.92<br />
5.5<br />
409 0.74 0.54<br />
570 0.83 0.63<br />
244 1.28 0.97<br />
7.0<br />
409 0.87 0.62<br />
570 1.02 0.75<br />
244 1.40 1.08<br />
9.0<br />
409 0.92 0.67<br />
570 1.32 1.00<br />
*Represent the Molar absorptivity<br />
multiplied by the cell width<br />
aA/aG<br />
1.29<br />
1.37<br />
1.32<br />
1.32<br />
1.40<br />
1.36<br />
1.30<br />
1.37<br />
1.32<br />
Figure 2: standard curve of arginine and<br />
glycine colored complexes with ninhydrin<br />
at different pH values measured at 570 nm<br />
35<br />
Figure 3: uv-visible spectra of arginine and<br />
glycine colored complexes with ninhydrin at<br />
different pH values<br />
According to the non-aqueous titration<br />
method; the titration end point of the titration<br />
curve between standard perchloric acid with<br />
the complex was determined<br />
potentiometrically. A typical plot for the<br />
titration curve was exhibited in figure ( 4 )
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
Figure 4: titration curve between standard<br />
perchloric acid (0.1N) and arginine<br />
acetylsalicylate –glycine complex.<br />
The result of stability study indicates the<br />
appearance of three spots on TLC at (50, 60,<br />
79)ºC and only one spot at (25, 40) ºC as<br />
summarized in table (4).<br />
Table 4: *Rf values of the arginine<br />
acetylsalicylate – glycine complex at different<br />
temperatures for certain time intervals<br />
C Intial 1 st 2 nd 4 th<br />
25 0.42 0.42 0.42 0.41<br />
40 0.42 0.42 0.42 0.41<br />
50 0.42 0.43 0.46 0.55<br />
60 0.42 0.44 0.45 0.57<br />
70 0.42 0.45 0.55 0.56<br />
*The solvent system is (n-propanol : 34%<br />
ammonia 7 :3 )<br />
Interference<br />
As mentioned earlier the degradation<br />
product of arginine acetylsalicylate – glycine<br />
complex might interfere with the<br />
determination of arginine acetyl Salicylic acid<br />
and glycine by the proposed method. The<br />
evacuation step in the acetylsalicylic acid<br />
analysis procedure ensures complete removal<br />
of acetic acid thus prevents its influence on the<br />
titration of acetyl salicylic acid with sodium<br />
hydroxide. Beside, the determination of the<br />
amount of salicylic acid (20) and the subsequent<br />
correction eliminates its interference on the<br />
acetyl Salicylic acid determination. The non –<br />
aqueous titration procedure is not affected by<br />
all the degradation procedure as this procedure<br />
is selective for basic groups such as the amino<br />
group.<br />
Conclusion<br />
The proposed procedure is suitable to be<br />
used as a quality control procedure for the<br />
36<br />
determination of arginine acetylsalicylate –<br />
glycine complex, as well as many formulas<br />
that contain essential and non essential amino<br />
acids (free or in combination)in different<br />
preparation like food supplement or other<br />
pharmaceutical preparation.This proposed<br />
procedure is simple, fast and accurate in<br />
compares with other procedures.<br />
Refrences<br />
1. Xie Y., Wang T. and Goodeng R.,<br />
Xuexiao Huaxue Xuebao, 1980; 14(2) :<br />
175 -179.<br />
2. Kiungel OH.,Ensing K., Hegge H., Franke<br />
JP. And Zeeuw D., J Planer Chromatogrmod<br />
TLC, 1933;6(2):112-116.<br />
3. Kmeted VJ., Pharm Biomed Anal., 1992;<br />
10(10-12):1073-1076.<br />
4. Edgar E. Themer and Emil W. Cinrczak, J<br />
Pharm Sci, 2006; 66(1):139-140.<br />
5. Britsh Pharmacopoeia, The Stationery<br />
Offices, London, 2008:192-193.<br />
6. Juskowiak B., Spectrochim Octa, 1993;<br />
49(2): 173-182.<br />
7. Erdal Dinc, Abdil Ozdemir and Dumitru<br />
Baleanu, Talanta, 2005; 65(1):36-47.<br />
8. Erdal Dinc, , Talanta,1999;48(5):1145-<br />
1157.<br />
9. Abbas Afghani and Morteza Bahram,<br />
,Talanta, 2005;66(3):712-720.<br />
10. D.C.Harris, Quantitative Chemical<br />
Analysis, 6th Ed., 2003:130, 15.<br />
11. Sexberg H. and Kowalski R., Anal Chem,<br />
1979; 51:1031.<br />
12. Kalivas H. and Kowalski R., Anal Chem,<br />
1981; 53:2212.<br />
13. Margaret Raymond, Clemens Jachum and<br />
Bruce R. Kowalski, J Chem Fdu, 1988;<br />
60(12):1072-1073.<br />
14. Michael Berglund and Douglas C. Baxter,<br />
J Microchimica Acta, 1955; 119(3-4):311-<br />
312.<br />
15. Marjanovic L,McCrindle RI,Botha<br />
BM,Potgieter HJ,Anal.bioanal chem,<br />
2004; 379(1):104-107.<br />
16. Matschat R,Hassler J,Traub H,Dette<br />
A,Anal Bioanal chem,2005;383(7-<br />
8):1060-1070.<br />
17. Ulberth F, Anal Bioanal chem, 2006;<br />
386(4):1121-1136.<br />
18. Ignacio Ferrandez-Figares,Luis Cuadros<br />
Rodriguez,Antonio Gonzalez-Casado,j of<br />
chromatography B,2004;799:73-74.<br />
19. L.Cuadrose Rodriguez,L.Gawiz Gracia,<br />
E.M.Amansa Lopez,J.M.Bosque Sandra,<br />
Trends Anal chem.,2001(20):620.<br />
20. N.R.Meyckik, Y.U.Nikolaeva, I.P.<br />
Ermakov , Biochemistry (Moscow), 2009;<br />
74 (8): 933-937.
Iraqi J Pharm Sci, Vol.19(2) 2010 Modification of GSAM<br />
21. Herida R.N. Marowa,Cristian<br />
C.C.Lopes,Simone G.Cardoso ,Lat.Am. J.<br />
Pharm., 2003;22(4):339-342.<br />
22. Yamamoto M,Taguchi K,Journal of The<br />
Japanese Association for Petroleum<br />
Technology,2000;65(5)469-476.<br />
23. Leigh M.Clemow,W.Roy Jackson, Fuel,<br />
2002; 81(7):959-961.<br />
24. Ieggli, Carine Viana Silva; Cardoso,<br />
Simone Goncalves; Belle, Luziane Potrich<br />
, Journal of AOAC International , 2005;<br />
September 1.<br />
25. Sheng Yun LI, Yin Zhe REN, Feng Lin<br />
ZHAO, Chinese Chemical Letters, 2006;<br />
17( 8): 1065-1068.<br />
26. J. Demeester, M. Bracke, R. Vochten, A.<br />
Lauwers,j pharm sci,2006;67(5):729-730 .<br />
27. D. Kowalczuk, R. Pietraś, J. Baran,<br />
Annales Universitatisaari Aecurie-<br />
37<br />
sklodowska Lublin-Polonia, 2007,VOL.<br />
XX, N 2, 11:83-87.<br />
28. British Pharmacopoeia, the Stationery<br />
Offices, London, 2008:vol.4.A87.<br />
29. Jin X.,Yaovw Fenxi Zazhi,1992; 12(3):<br />
169-170<br />
30. Pin HS. Organic Chemistry,5th Ed., MC<br />
Graw-Hill,Inc,USA,1988:817,823.<br />
31. Holme J. and Peck H., Analytical<br />
Biochemistry,2nd ed., Longman Group,<br />
UK, 1933;49:384-396.<br />
32. Mihajlovic R., Anal Chimicia Acta, 1990;<br />
229:287-290.<br />
33. Skoog AD.,West MD. And Holler JF.<br />
Fundamentals of Analytical Chemistry,<br />
5th ed., WB-Saunders Company, USA,<br />
1988:249.<br />
34. Kontoyannis G., J Pharm Biomedical<br />
analysis, 1993; 13(1):73.
Iraqi J Pharm Sci, Vol.19(2) 2010 Lithotripsy of urinary stone by Carum copticum seeds<br />
Lithotripsy of Different Urinary Tract Stones by Using Seeds of<br />
Carum copticum<br />
Ahmed G. Sabar* ,1<br />
* Department o f Community Health ,College of Health and Medical Technology, Baghdad,Iraq .<br />
Abstract<br />
It has been a well-known practice to use seeds and the essential oil of Carum copticum as a<br />
strongly antiseptic , antispasmodic , aromatic , bitter , diaphoretic , digestive , diuretic ,<br />
expectorant and tonic. Also used for cure influenza, asthma, and rheumatoid arthritis. To our<br />
knowledge it will be the first time to use the seeds of this herb as a urinary tract stone lithotripsy.This<br />
research aimed to the use of these seeds as a lithotripsian against different types of urinary stones and<br />
determine the efficiency of these preparation against which types of stone.A liquid solution was<br />
prepared from dissolving the seeds powder in cow milk and then concentration this preparation was<br />
done by boiling at 100 ° C to reduce the volume of solution to the half.The treatment was given via oral<br />
administration for successive 9 days before breakfast. 350 patients with urinary stone of different type<br />
took part in this research. All patients were subjected to ultrasonography and intravenous pyelography<br />
examinations to localized the position and detect diameter of stone. The above examination and also<br />
biochemical tests for diagnosis of stones ingredients were repeated after the administration of treatment<br />
and excretion of stone fragments in urine. The results were so promising especially against pure caoxalate<br />
stone.<br />
Key words: Carum copticum; Lithotripsy; Ca-Oxalate stone; mixed stone<br />
ﺔﺻﻼﺨﻟﺍ<br />
, ﺓﺭﺪﻣ , ﺺﻐﻤﻠﻟ ﺓﺩﺎﻀﻣ ﺩﺍﻮﻤﻛ ﺭﻭﺬﺒﻟﺍ ﻦﻣ ﺔﺼﻠﺨﺘﺴﻤﻟﺍ ﺓﺭﺎﻴﻄﻟﺍ ﺕﻮﻳﺰﻟﺍ ﻚﻟﺬﻛﻭ ( ﻲﻛﻮﻠﻤﻟﺍ ﻥﻮﻤﻛ)<br />
ﺓﻮﺨﻨﻟﺍ ﺕﺎﺒﻧ ﺭﻭﺬﺑ ﺖﻣﺪﺨﺘﺳﺍ<br />
ﺍﺬﻫ ﺭﻭﺬﺑ ﺎﻨﻣﺪﺨﺘﺳﺍﻭ . ﻲﻣﺰﺗﺎﻣﻭﺮﻟﺍ ﻞﺻﺎﻔﻤﻟﺍ ﺏﺎﻬﺘﻟﺍﻭ ﻝﺎﻬﺳﻻﺍﻭ ﻮﺑﺮﻟﺍﻭ ﻝﺎﻌﺴﻟﺍﻭ ﺩﺮﺒﻟﺍ ﺝﻼﻌﻟ ﺎﻀﻳﺍ ﺖﻠﻤﻌﺘﺳﺍﻭ , ﺔﻌﺸﻘﻣ , ﻢﻀﻬﻠﻟ ﺓﺪﻋﺎﺴﻣ<br />
ﺕﺎﺒﻧ ﺭﻭﺬﺑ ﻡﺍﺪﺨﺘﺳﺍ ﻰﻟﺍ ﺚﺤﺒﻟﺍ ﺍﺬﻫ ﻑﺪﻬﻳ . ﻝﺎﺠﻤﻟﺍ ﺍﺬﻫ ﻲﻓ ﺓﺮﻣ ﻝﻭﻻ ﺎﻨﺗﺎﻣﻮﻠﻌﻣ ﺐﺴﺣ ﺔﻴﻟﻮﺒﻟﺍ ﻱﺭﺎﺠﻤﻟﺍ ﻰﺼﺣ<br />
ﺖﻴﺘﻔﺘﻟ ﺝﻼﻌﻛ ﺕﺎﺒﻨﻟﺍ<br />
ﻢﺗ . ﻰﺼﺤﻟﺍ ﻉﺍﻮﻧﺍ ﻦﻣ ﻉﻮﻧ ﻱﺍ ﻩﺎﺠﺗ ﺮﺒﻛﻻﺍ ﺔﻴﻟﺎﻌﻔﻟﺍ ﺪﻳﺪﺤﺗﻭ , ﺔﻔﻠﺘﺨﻤﻟﺍ ﺎﻬﻋﺍﻮﻧﺎﺑ ﺔﻴﻟﻮﺒﻟﺍ ﻱﺭﺎﺠﻤﻟﺍ ﻰﺼﺣ ﺖﻴﺘﻔﺘﻟ ﺝﻼﻌﻛ ﺔﻳﺪﻨﻬﻟﺍ ﺓﻮﺨﻨﻟﺍ<br />
ﻰﻟﺍ ﺔﻴﻤﻜﻟﺍ ﻝﺍﺰﺘﺧﻻ ﺔﻳﻮﺌﻣ ﺔﺟﺭﺩ 100 ﻰﻟﺍ ﻪﻧﺎﻴﻠﻐﺑ ﻊﻴﻘﻨﻟﺍ ﺰﻴﻛﺮﺗ ﻢﺗﻭ ﺭﺎﻘﺑﻻﺍ ﺐﻴﻠﺣ ﻡﺍﺪﺨﺘﺳﺎﺑ<br />
ﺕﺎﺒﻨﻟﺍ ﺭﻭﺬﺑ ﻕﻮﺤﺴﻣ ﻦﻣ ﻊﻴﻘﻧ ﺮﻴﻀﺤﺗ<br />
ﺩﻮﺟﻭ ﻦﻣ ﻥﻮﻧﺎﻌﻳ ﻦﻤﻣ ﺎﻀﻳﺮﻣ 350 ﺔﺳﺍﺭﺪﻟﺍ ﻩﺬﻫ ﻲﻓ ﻙﺭﺎﺷ.<br />
ﺭﺎﻄﻓﻻﺍ ﻞﺒﻗ ﺔﻴﻟﺎﺘﺘﻣ ﻡﺎﻳﺍ 9 ﺓﺪﻤﻟ ﻢﻔﻟﺍ ﻖﻳﺮﻃ ﻦﻋ ﻂﻴﻠﺨﻟﺍ ﻲﻄﻋﺍ . ﻒﺼﻨﻟﺍ<br />
ﻢﺛ , ﻰﺼﺤﻟﺍ ﻢﺠﺣﻭ ﻊﻗﻮﻣ ﺪﻳﺪﺤﺘﻟ ﺔﻧﻮﻠﻤﻟﺍ ﺔﻌﺷﻻﺍﻭ , ( ﺔﻴﺗﻮﺼﻟﺍ ﻕﻮﻓ ﺕﺎﺟﻮﻤﻟﺍ)<br />
ﺭﺎﻧﻮﺴﻟﺍ ﺕﺎﺻﻮﺤﻓ ﻰﻟﺍ ﺍﻮﻌﻀﺧﺍ ﻲﻟﻮﺒﻟﺍ ﻯﺮﺠﻤﻟﺍ ﻲﻓ ﻰﺼﺤﻟﺍ<br />
ﺔﻳﻭﺎﻴﻤﻴﻛﻮﻳﺎﺒﻟﺍ ﺕﺎﺻﻮﺤﻔﻟﺍ ﺖﻳﺮﺟﺍ ﻚﻟﺬﻛ ﺝﻼﻌﻟﺍ ﻡﺍﺪﺨﺘﺳﺍ ﺪﻌﺑ ﺕﺎﺻﻮﺤﻔﻟﺍ ﻩﺬﻫ ﺕﺪﻴﻋﺍ.<br />
ﻡﺎﻌﻟﺍ ﺭﺍﺭﺩﻻﺍ ﺺﺤﻓ ﺚﺤﺒﻟﺍ ﺕﺎﻨﻴﻋ ﺔﻓﺎﻜﻟ ﻱﺮﺟﺍ<br />
ﺕﻻﺰﻛﻭﺍ ﺓﺎﺼﺣ ﻩﺎﺠﺗ ﺔﻴﻟﺎﻋ ﺓءﺎﻔﻜﺑ ﻝﺎﻌﻓ ﺝﻼﻌﻟﺍ<br />
ﺍﺬﻫ ﻥﺍ ﻦﻴﺒﺗ ﺚﻴﺣ ﺎﻬﺗﺎﻧﻮﻜﻣ ﺺﻴﺨﺸﺘﻟ ﺭﺍﺭﺩﻻﺍ ﻲﻓ ﺔﻟﺯﺎﻨﻟﺍﻭ ﺔﺘﺘﻔﺘﻤﻟﺍ ﺕﺎﻴﺼﺤﻟﺍ ﻰﻠﻋ<br />
. ﻯﺮﺧﻻﺍ ﻉﺍﻮﻧﻻﺍ ﻦﻣ ﺕﺍﻮﺼﺤﻠﻟ ﻞﻗﺍ ﺔﺟﺭﺪﺑﻭ ﺔﻴﻘﻨﻟﺍ ﻡﻮﻴﺳﺎﺗﻮﺒﻟﺍ<br />
Introduction<br />
Renal stones (nephrolithiasis) are<br />
concretion composed of crystalline<br />
components and organic Matrix [1]. Although the<br />
symptomatic presentations may be similar ;the<br />
disorder is heterogeneous as to composition<br />
and etiology.Today, most urinary stones in<br />
patients in most countries are renal stones.<br />
About 1-4% of the population is believed to<br />
have kidney stones every year in USA and<br />
Europe. About 2-5% of population in Asia, 8-<br />
15% in Europe and North America and 20% in<br />
saudia Arabia develop kidney stone in their<br />
lifetime [2, 3, and 6] . Renal stones tend to recur,<br />
and the rate of recurrence is about 75% during<br />
20 years environmental and genetic factors [4-5] .<br />
A specific diagnosis for every patient with<br />
kidney stones, may give very important<br />
information about the stone-formation<br />
mechanism and the pharmaceutical manner to<br />
38<br />
[5-6]<br />
prevent recurrent stone formation<br />
.Carum copticum with herbarium number 293-<br />
0303-1 is a plant in Umbelliferae family with a<br />
white flower and small, brownish seeds. This<br />
plant is commonly grows in India,Iran,Egypt<br />
and Europe [7] .The seeds and especially the<br />
essential oil are strongly antiseptic,<br />
antispasmodic, diuretic, and used in the<br />
treatment of so many diseases.The seeds<br />
contains about 4-6% essential oil, of which 45-<br />
55% is thymol [8] , while the essential oil in the<br />
dried fruits (2.5-5% ) is dominated by thymol<br />
(35-60%) [9] .From South India Carum copticum<br />
fruits, almost pure thymol has been isolated<br />
(98%) , but the leaf oil was found to be<br />
composed of monoterpenoids and<br />
sesquiterpenoids: 43%cadinene, 11%<br />
longifolene, 5% thymol, 3% camphor and<br />
others [9] . The effective components of this<br />
1Corresponding author E- mail : ahmedalqaicy@yahoo.com , ahmed@hotmail.com<br />
Received : 16/11/2009<br />
Accepted : 1/8/2010
Iraqi J Pharm Sci, Vol.19(2) 2010 Lithotripsy of urinary stone by Carum copticum seeds<br />
Plant , responsible for the observed<br />
bronchodialatory effect [6] ,despite the availability<br />
of modern medication the propensity towards the<br />
traditional medications is growing through out<br />
the word [7,8] which needs scientific investigation<br />
for evaluating the therapeutic effects and their<br />
mechanism of action. Indeed no acute toxicity<br />
data were available for Carum copticum<br />
,although animal studies of putative beneficial<br />
effect of this plants seeds involved the use of a<br />
dose of 500mg/kg body weight in mice and rat<br />
without morbidity or mortality ascribable to the<br />
herb, suggesting that the oral LD50 of Carum<br />
copticum (fruit/seeds) is likely to be higher than<br />
that figure [9] . The present study was carried out to<br />
determine the role of Carum copticum seeds as a<br />
herbal medication for treatment urinary tract<br />
stones.<br />
Materials and Methods<br />
The study of effects of Carum copticum<br />
seeds as a lithotripsian agent was carried out in<br />
2008 on (350) patients diagnosed by specialist<br />
physician in private clinic from different sites of<br />
Iraq (Baghdad,Diala,Mousol) and the<br />
experimental part was undertaken during the<br />
period (2001-2008).<br />
Materials<br />
Seeds of Carum copticum (other Latin<br />
name : Trachyspermum ammi ), were collected<br />
fro m local market in Baghdad, identified under<br />
expert guidance ,taxonomy was performed by<br />
national herbarium of Iraq at 1997,depended on<br />
Ayurvedic pharmacopoeia of India (API) [10] ,<br />
and kew herbarium [11] , liquid milk, sugar. The<br />
dose normally recommended in traditional<br />
Ayurvedic use is 3-6 gm / day , presumably<br />
being 3 gm once or twice a day [12] . Also this<br />
dose was documented in Arabic antique<br />
manuscript (Tathkarat Daood AL-Antaky) [13] .<br />
Methods<br />
Before started giving the preparation we<br />
obtained the written consent of the patients who<br />
included in the study; (15gm) of seeds were<br />
ground to a very fine powder.(Total dose for<br />
each patients taken with in 9 days) ;(5gm) o f<br />
grounded seeds were boiled with 150 ml of liquid<br />
cow milk and 25 gm sugar, until half of the<br />
volume was obtained; the preparation was kept<br />
cool [13] ;This preparation divided to ( 3 ) equal<br />
doses, each patients was given single dose a day<br />
before breakfast for a period of 3 days ; This<br />
procedure was repeated for remainder (10gm) of<br />
grounded seeds ; Ultrasonography (U /S) and<br />
Intravenous pyelography (IVP), were performed<br />
pre-, and post treatment to be sure of curing<br />
urinary tract from any stone; Urinary tract stones<br />
were of different sizes ranging from 5mm and<br />
1.2 cm and they were seen in the kidney (renal<br />
stone) at upper pole calyx, mid renal part and<br />
39<br />
lower pole calyx, also they were seen in the<br />
ureter (uretral stones) , and finally they were seen<br />
inside urinary bladder (vesical stones) . In<br />
addition general urine examination (GUE) was<br />
done for all patients ; Qualitative analysis of<br />
stone / fragments passed after herbal treatment, a<br />
procedure described by Hodgkinson [14] , was<br />
employed to figure out the chemical constituent<br />
of urinary stone<br />
Results<br />
The present study included (350) patients<br />
with different urinary tract stones were treated<br />
with liquid extract of Carum copticum seeds.<br />
Table 1 : Chemical constituent of stones<br />
Type of stone<br />
Pure Ca-oxalate<br />
(Ca-oxalate/uric<br />
acid) mixed<br />
stone<br />
( Ca-oxalate/<br />
Hydroxyapatite)<br />
mixed stone<br />
Total<br />
No. of<br />
patients<br />
170<br />
100<br />
80<br />
350<br />
Table 2 : Lithotripsy events<br />
Type of stone<br />
Pure ca-oxalate<br />
(Ca-oxalate./<br />
Uric acid)<br />
mixed stone<br />
( Ca-oxalate./<br />
hydroxyapatite)<br />
mixed stone<br />
Total<br />
No. of<br />
patie nts<br />
170<br />
100<br />
%<br />
48,57<br />
28.57<br />
22,86<br />
100%<br />
No. of<br />
lithotripsed<br />
stone<br />
170<br />
53<br />
Table 3 : Duration of lithotripsy<br />
Pure ca-oxalate<br />
80<br />
350<br />
Type of stone<br />
Ca-oxa./uric acid)(Mixed stone.<br />
25<br />
Ca-oxalate./hydroxyapatite) Mixed stone.<br />
Patients age<br />
range (years)<br />
(20-45)<br />
( 20-50)<br />
(22-50)<br />
%<br />
100<br />
53<br />
31.25<br />
Time<br />
(days)<br />
2-7<br />
7-12<br />
7-15
Iraqi J Pharm Sci, Vol.19(2) 2010 Lithotripsy of urinary stone by Carum copticum seeds<br />
Table 4 : Response the types of stones to the<br />
treatme nt with Carum copticum<br />
Type of stone<br />
Pure ca-oxalate.<br />
Ca-oxalate./uric<br />
acid mixed stone.<br />
(1)<br />
Caoxa./hydroxyapatite<br />
mixed stone. (2)<br />
Response<br />
(%)<br />
170<br />
(100%)<br />
53<br />
(53%)<br />
25<br />
(31.25%)<br />
* H.S: Highly Significant<br />
C.S (p-value)<br />
Be tween each pairs of<br />
types<br />
Ca-oxa.×mixe d(1)<br />
Ca-oxa.×mixe d(2)<br />
mixe d(1)×mixed(2)<br />
0.000<br />
*H.S<br />
0.000<br />
H.S<br />
0.003<br />
H.S<br />
Discussion<br />
The results of this study including the<br />
investigation of the efficiency of Carum<br />
copticum seeds (liquid solution), locally prepared<br />
on urinary stone among (350) patients.The<br />
results present in table (1) showed that pure caoxalate<br />
consist the larger percentage among types<br />
of stones. Since a (170) patients out of (350)<br />
patients (48.57%) have a pure ca-oxalate urinary<br />
stone.Results of table (1) revealed that (28.57),<br />
(22.86) of patients got mixed stone (caoxalate\uric<br />
acid) and (ca-oxalate<br />
\hydroxyapatite) respectively.The results cleared<br />
out in table (2) indicated that the local<br />
preparation of Carum copticum seeds have a<br />
good affectivity on ca-oxalate stones , since a<br />
hundred percent of this type of urinary stone had<br />
been lithotripsed , comparing to (53%) for mixed<br />
(ca-oxalate\uric acid) and (31.25%) for mixed<br />
(ca-oxalate\hydroxyapatite) . Recently in India<br />
had successfully purified an anticalcifying<br />
protein from the seeds of Carum copticum using<br />
oxalate depletion assay and deciphered its<br />
inhibitory activity against ca-oxalate crystals<br />
growth .The antilithiatic potential of Carum<br />
copticum was confirmed by its ability to maintain<br />
renal functioning, reduce renal injury and<br />
decrease crystal excretion in urine and retention<br />
in renal tissue [15] .It was obvious from the results<br />
presented in table (3) that pure ca-oxalate<br />
required the minimum duration to complete<br />
lithotripsy. While mixed stone of both types<br />
required a longer time to complete lithotripsy.<br />
The response of types of stone was indicated in<br />
table (4) which showed that pure ca-oxalate was<br />
the most affected types of stones by the treatment<br />
with Carum copticum seeds compared with other<br />
two types of urinary stones mainly (caoxalate\uric<br />
acid) mixed stone and (caoxalate\hydroxyapatite)<br />
mixed stone. On the<br />
other hand type two of stone (ca-oxalate\uric<br />
40<br />
acid) mixed stone was affected by treatment with<br />
Carum copticum seeds more than the mixed<br />
stone (ca-oxalate\hydroxyapatite). Our results<br />
confirmed the antilithiatic properties of Carum<br />
copticum seeds that authorized by many<br />
researchers [16-19] . Biostatistical analysis<br />
(Binomial-test and Z-test) confirmed these<br />
results. It could be argued for that the effect of<br />
herb extract (crude extraction) is depending<br />
totally on chemical structure of urinary tract<br />
stones, also it is clear from the results presented<br />
in table (4) that ca-oxalate whenever existed as<br />
one of the constituent of urinary tract stones it<br />
will provoke or stimulate the action of herbs<br />
seeds extract, in other word ca-oxalate is decisive<br />
component of urinary tract stone that encourage<br />
lithotripsy whenever Carum copticum seeds<br />
extract are available.In this view, mixed stones<br />
which are either (ca-oxalate\uric acid) or (caoxalate\hydroxyapatite)<br />
showed different<br />
response to the treatment with Carum copticum<br />
seeds extraction and that in our view is<br />
corresponding to the amount of ca-oxalate<br />
present in the mixture .In general, the lithotripsic<br />
effects of Carum copticum seeds against urinary<br />
tract stones were mentioned by Arabic scientist<br />
sheikh Dawood Antaki (about 1008 A. H) [13] .<br />
Conclusion<br />
Thus, the present study suggests the<br />
potential of Carum copticum seeds in lithotripsy<br />
of urinary stone, especially calcium oxalate and<br />
forms the basis for the development of<br />
antilithiatic drug interventions against<br />
urolithiasis.<br />
Acknowledgment<br />
The author is grateful to Dr. Nabeel Fadel ,<br />
Dr. Nasseer Abed Agha, and Dr. Akram Mohd<br />
Al-mahdawy, for help in the diagnosis of the<br />
caseas. Thank also to Dr. Zuhair Nouman Al-Ani<br />
a Professor of pharmacogenetic for his scientific<br />
advices.<br />
References<br />
1. Pak CYC. Kidney stones. Williams Textbook<br />
of Endocrinology 1992; 1519-36.<br />
2. Pak CYC.Kidney stones. Lancet 1998; 351:<br />
1797-801.<br />
3. Kamoun A, Daudon M, Abdelmoula J,<br />
Hamzaoui M,Chouachi B, Houissa T, Zghal<br />
A, Ben Ammar S, Belkahia C, Lakhoua R.<br />
Urolithiasis in Tunisian Children. Pediater<br />
Nephrol 1999; 13(9):920-5.<br />
4. Gault MH, Chafe L. Relationship of<br />
frequency, age, sex, stone weight and<br />
composition in 15 624 stones. J Urol 2000; 64<br />
(2): 302-7.<br />
5. Kourambas J, Aslan P, The CL, Mathias BJ,<br />
Preminger M. Role of stone analysis in<br />
metabolic evaluation and medical treatment of
Iraqi J Pharm Sci, Vol.19(2) 2010 Lithotripsy of urinary stone by Carum copticum seeds<br />
nephrolithiasis. J Endocrinol 2001; 15 (2):<br />
181-6.<br />
6. Thomas SE, Stapleton FB. Leave no (stone)<br />
untreated; understanding the genetic bases of<br />
calcium-containing urinary stones in<br />
childhood. Adv Pediatr 2000; 47: 199- 221.<br />
7. Seyed Hassan Hejazian; Mohd Hossein ,and<br />
Mohd Dashti,.(Antinociceptive effect of<br />
Carum copticum extract in mice using<br />
formalin test).,world Appl.Sci.J.<br />
2008,3(2):215-219<br />
8. Chopra. R. N., Nayar. S. L. and Chopra. I. C.<br />
Glossary of Indian Medicinal Plants<br />
(Including the Supplement). Council of<br />
Scientific and Industrial Research, New Delhi.<br />
1986.<br />
9. Versita,Warsaw. Fumigant toxicity of<br />
essential oil from Carum copticum against<br />
Indian meal moth,journal of plant protection<br />
Research,Dec. 2008, 48(4);1427- 4345.<br />
10. Ayurvedic Pharmacopoeia of India,A<br />
jamoda.Government of india.Part 1980;<br />
Vol.I,First Edition:2-3.<br />
11. Bull.of Miscellaneous information(Royal<br />
Garden,Kew), 1931 ; 1931(6); 299- 344.<br />
12. The Ayurvedic Pharmacopoeia of<br />
India,Appendix.,Government of india.Part<br />
I,Volume II,First Edition: 1999; 189-93.<br />
13. Shaikh Dawood Antaki. Tadhkirat Uli Al<br />
Albab Wal jamia lil Ajab Alujab. Medieval<br />
medical book. 10 th century, product code :<br />
1568.<br />
41<br />
14. Hodgkinson A. A combined qualitative and<br />
quantitative procedure for the chemical<br />
analysis of urinary tract calculi. J.clin.path.<br />
1971, 24,147-151.<br />
15. Tan Zeer Kaur , Rakesh, et al., In vivo<br />
efficacy of Trachyspermum ammi<br />
anticalcifying protein in urolithiatic rat model.<br />
Jour. of Ethnopharmacology .2009, 126(3)<br />
459-462.<br />
16. A k Pathak, N Nainwal et al., Pharmacological<br />
activity of Trachyspermum ammi : A review.<br />
Jour.of Pharmacy Research, 2010; 3(4); 895-<br />
899.<br />
17. Gurinder J and Daljit S.A., Bioactive potential<br />
of Anethum graveolens,Foeniculum vulgare<br />
and Trachyspermum ammi belonging to the<br />
family Umbelliferae-current status. Jor. Of<br />
Medicinal plant research 2010; 4(2), 87-94.<br />
18. Shazia S. ,Mir A.K, Mushtaq A. and<br />
Muhammed Z., Indigenous Knowledge of folk<br />
medicines by the women of District chakwal,<br />
Pakistan . Jor.Ethnobotanical Leaflets 2006;<br />
10: 243-253.<br />
19. Muhammed H.,Sumera A.,Mir Ajab K,<br />
Ethnopharmacology, indigenous collection<br />
and preservative technique of some frequently<br />
used medicinal plants of utror and<br />
gabral,district swat,Pakistan. Afric.Jor.of<br />
Tradition. ,Complem.,and Altenet. Med. 2006<br />
; 3(2) , 57-73.
Iraqi J Pharm Sci, Vol.19(2) 2010 Stability of Cfm and Cfz with CA against β- lactamase<br />
Evaluation of Stability of Cefamandol and Ceftazidime with<br />
Clavulanic Acid Against Extended Spectrum β- Lactamase<br />
Siham S. Shaokat * and Hamoudi A. Hameed* ,1<br />
* Ministry of Industry and Minerals, Food and Drugs Sector, Baghdad, Iraq.<br />
Abstract<br />
The aim of this study is to evaluate in-vitro activity of Cefamandol (Cfm) and Ceftazidime (Cfz),<br />
in combination with Clavulanic acid (CA) against ten complicated multiresistant uropathogenic E.coli<br />
.One hundred clinical strains were isolated from patients with chronic urinary tract infections (UTIs),<br />
these isolates were identified by the Api identification systems. The antimicrobial susceptibility tests<br />
were determined by Kirby-Bauer method, all of them were sensitive to Imipenem (Imp). Ten strains<br />
were chosen for the present study, they were resistant to Ampicillin (Amp), Amoxicillin (Amo),<br />
Carbenicillin (Cb), Ticarcillin (Tic), Azlocillin (Azl), Amoxicillin\ Potassium Clavulanate<br />
{Augmentin(Amc)}, (Amo\CA), Ticarcillin\ Potassium Clavulanate {Timentin} (Tic\CA) ,Cefazolin<br />
(Cfo) ,Cefaloridin (Cfr), Cefamandol, (Cfm),Ce foxitin, Ceftazidime (Cfz), Cefixime (Cxm),<br />
Cefoperazone( Cfp) and Aztreonam (Atm), also resistant to other antibiotics,<br />
Tetracycline(Tc),Cloramphenicol(Cm),Gentamycin(G),Amikacin (Amk), Ciprofloxacin (Cip) and<br />
Trimethoprim. 50% of the isolates were resistant to Nalidixic acid and Rifampicin. The minimum<br />
inhibitory concentrations of Cefamandol and Ceftazidime were determined, by tube method. Transfer<br />
of plasmids were done by direct conjugation test to sensitive standard E.coli ,cell free β- lactamases<br />
were prepared and detected by macro-iodometric method. The activity of each cell free ß – lactamases<br />
extract against Cfm and Cfz were determined by disks diffusion method (microbiological Masuda<br />
method) .Excellent activities were obtained against these strains when Cfm and Cfz, combined with<br />
CA, therefore complete zones of inhibition were obtained indicated the prevalence of extended<br />
spectrum β- lactamases in E.coli. The stability of Cfm and Cfz in the presence of CA were useful in the<br />
treatment of chronic urinary tract infections caused by multiresistant β- lactamase (ESBL) producer<br />
E.coli.<br />
Key words: Exte nde d spectrum β- lactamases, Imipe nem, Aztreonam, Ce ftaz idime .<br />
ﺔﺻﻼﺨﻟﺍ<br />
E- ) ﻩﺎﺠﺗ ﻚﻧﻻﻮﺠﻓﻼﻜﻟﺍ ﺾﻣﺎﺣ ﺔﻓﺎﺿﺎﺑ ( Cfz)<br />
ﻢﻳﺩﺯﺎﺘﻔﻴﺴﻟﺍﻭ ( Cfm)<br />
ﻝﻭﺪﻨﻣﺎﻔﻴﺴﻟﺍ ﺔﻴﻟﺎﻌﻓ ﺔﺳﺍﺭﺩ ﻰﻟﺍ ﺚﺤﺒﻟﺍ ﻑﺪﻬﻳ<br />
ﻥﺍ ﺔﺳﺍﺭﺪﻟﺍ ﺖﻨﻴﺑ ﺪﻗﻭ ﻦﻣﺰﻤﻟﺍ ﺔﻴﻟﻮﺒﻟﺍ ﻱﺭﺎﺠﻤﻟﺍ ﺏﺎﻬﺘﻟﺍ ﻦﻣ ﻥﻮﻧﺎﻌﻳ ﻰﺿﺮﻣ ﻦﻣ ﺔﻟﻭﺰﻌﻣ ﻡﺎﺘﻛﻻﺎﺘﻴﺒﻟﺍ ﺕﺍﺩﺎﻀﻣ ﺔﻋﻮﻤﺠﻤﻟ ﺔﻣﻭﺎﻘﻤﻟﺍ ( Coli<br />
ﻢﻳﺩﺯﺎﺘﻔﻴﺴﻟﺍﻭ ﻝﻭﺪﻧﺎﻣﺎﻔﻴﺴﻟﺍ ﻦﻣ ﻞﻛ ﺔﻴﺗﺎﺒﺛ ﻥﺍﻭ ﻚﻧﻻﻮﺠﻓﻼﻜﻟﺍ ﺾﻣﺎﺣ ﺩﻮﺟﻮﺑ ﻥﻮﻜﺗ ﻢﻳﺩﺯﺎﺘﻔﻴﺴﻟﺍﻭ ﻝﻭﺪﻧﺎﻣﺎﻔﻴﺴﻟﺍ ﻦﻣ ﻞﻜﻟ ﻰﻠﺜﻤﻟﺍ ﺔﻴﻟﺎﻌﻔﻟﺍ<br />
ﺕﺍﺩﺎﻀﻤﻟ ﺔﻣﻭﺎﻘﻤﻟﺍ ( E-Coli ) ﻦﻋ ﺔﺠﺗﺎﻨﻟﺍ ﺔﻴﻟﻮﺒﻟﺍ ﻱﺭﺎﺠﻤﻟﺍ ﺏﺎﻬﺘﻟﺍ ﺔﺠﻟﺎﻌﻣ ﻲﻓ ﺎﻤﻬﻣﺍﺪﺨﺘﺳﺍ ﺔﻴﻧﺎﻜﻣﺍ ﻰﻟﺍ ﺮﻴﺸﺗ ﺾﻣﺎﺤﻟﺍ ﺩﻮﺟﻮﺑ<br />
. ﺚﺤﺒﻟﺍ ﻲﻓ ﺖﻣﺪﺨﺘﺳﺍ ﻲﺘﻟﺍ ﺺﻴﺨﺸﺘﻟﺍﻭ ﻢﻴﻴﻘﺘﻟﺍ ﻖﺋﺍﺮﻃ ﻝﻼﺧ<br />
ﻦﻣ ﻡﺎﺘﻛﻻﺎﺘﻴﺒﻟﺍ<br />
Introduction<br />
Clavulanic acid is a β- lactam;<br />
structurally it differs from Pnicillins in two<br />
respects, the replacement o f sulfur in the<br />
Penicillin thiazolidine ring with oxygen in the<br />
clavam oxazolidine ring and the absence of the<br />
side chain at position 6. Clavulanic acid a<br />
naturally occurring clavam isolated from<br />
Streptomyces clavuligerus has poor<br />
antibacterial activity but exerts a potent and<br />
irreversible inhibitory effect on β- lactamases<br />
especially penicillinase by blocking the active<br />
sites of these enzymes and is strongly<br />
synergistic with most o f the β- lactamines in<br />
vitro (1) lactam antibiotics<br />
. Due to this combination, Amoxicillin<br />
is protected from degradation and its spectrum<br />
is therefore extended to include bacteria<br />
normally resistant to amoxicillin and other β-<br />
(2) . In the case of β- lactam<br />
resistant bacteria a bacterial enzyme, β-<br />
lactamase, cleaves the β- lactam ring and<br />
renders the antibiotic inactive. β-lactamases<br />
are a large and diverse group of enzymes in<br />
which four clinically relevant classes are<br />
known (3) . β-lactamase continues to be the<br />
leading cause of resistance to β- lactam<br />
antibiotics among Gram-negative bacteria. In<br />
recent years there has been an increased<br />
incidence and prevalence of extendedspectrum<br />
β-lactamases (ESBLs), enzymes that<br />
hydrolyze and cause resistance to Oxyimino-<br />
Cephalosporins and Aztreonam.The majority<br />
of ESBLs are derived from the widespread<br />
broad - spectrum β- lactamases TEM-1 and<br />
SHV-1.<br />
1Corresponding author E- mail : hamodiabas@yahoo.com<br />
Received : 11/6/2009<br />
Accepted : 3/8/2010<br />
42
Iraqi J Pharm Sci, Vol.19(2) 2010 Stability of Cfm and Cfz with CA against β- lactamase<br />
ESBLs have become widespread throughout<br />
the world and are now found in a significant<br />
percentage of E.coli and Klebsiella<br />
pneumoniae strains in certain countries (4, 5, 6, 7) .<br />
There are also new families of ESBLs,<br />
including the Cefotaximase (CTX-M) and<br />
OXA- type enzymes, Ceftazidimase, as well as<br />
novel unrelated β- lactamases (8, 9, 10) . The<br />
stability of different Cephalosporins to the<br />
most important β- lactamases was assessed<br />
and many clinical studies have shown that up<br />
to 75% of the β- lactamases responsible for β-<br />
lactam resistance in G-negative bacteria were<br />
R-plasmid mediated (11) . Recently, new fourth<br />
generation cephalo-sporins, such as Cefepime,<br />
Cefpirome, Cefoselis, Cefditoren, Cefozopran<br />
(12) , were introduced into antibacterial<br />
chemotherapy and their activities were<br />
compared with other β-lactams such as<br />
Ceftazidime,Imipenem and Carbapenem,<br />
against..P.aeruginosa, Enterobacteriaceae<br />
(E.coli, Klebsiella pneumoniae) and Gpositive<br />
bacteria. In addition several drug<br />
combinations have been produced which<br />
contain both a β- lactam antibiotic and a β-<br />
lactamase inhibitor; the inhibitor has high<br />
affinity for β- lactamase, irreversibly binds to<br />
it, and thereby preserves the activity of the β-<br />
lactam. Currently, four penicillin inhibitor<br />
combinations are in clinical use: Ampicillin-<br />
Salbactam (Unasyn), Amoxicillin- Clavulanate<br />
(Augmentin), Ticarcillin –Clavulanate<br />
(Timentin) and Pipracillin- Tazobactam<br />
(Zosyn) (12) . Urinary tract infections (UTIs) are<br />
cause a significant health problem and E.coli<br />
has been reported to be the primary pathogen<br />
in approximately 80% of cases. E.coli, express<br />
structures called adhesins fimbriae or pili that<br />
help them bind to specific tissue (13) . The aims<br />
of the study are:<br />
1. To know the prevalence of extended<br />
spectrum β- lactamase (ESBL) in multi<br />
drug resistant (MDR) strains of E.coli<br />
isolated from complicated urinary tract<br />
infections.<br />
2. To evaluate the following combinations:<br />
Cefamandol/Clavulanateand Ceftazidime<br />
/ Clavulanate for their in vitro<br />
antimicrobial activity against complicated<br />
urinary tract infections caused by ESBLs<br />
β- lactamases.<br />
Materials and Methods<br />
Standard strains with plasmid – mediated<br />
beta – laectamases were used:<br />
1-E.coli K12 (TEM-1 type β- lactamase with<br />
isoelectric point 5.4) confer plasmid(R 111)<br />
and E.cloacae P99 ). 2-E.coli K12 (SHV-1<br />
type β- lactamase Pitton (type II) I.p 7.7<br />
(10).3-E.coli K12 600 Rif and E.coli K12 600<br />
43<br />
Nal Sensitive to antibiotics (10) . 4-Clinical<br />
isolates of E.coli. 5-Pure enzyme of Med Labs.<br />
6- E.coli ATCC 25922 provided by Medical<br />
city Identification of E.coli. A total of 100<br />
strains of E.coli were selected and identified<br />
by Api 20 E . System (Biomerieux vitek,<br />
Inc) (14) .<br />
Antibiotic susceptibility test (Disk diffusion<br />
method (10)<br />
The resistance pattern for antibiotics<br />
were determined by Kirby/Bauer diffusion<br />
assay on Mueller – Hinton agar (20ml / plate)<br />
the inoculum was 10 4 – 10 5 CFU / ml, of 6<br />
hours cultures at 37ºC for 24 hours.The<br />
antibiotics used were as follow: Amoxicillin<br />
(Amo) 30 µg, Augmentin(Amc) (Amo 20µg +<br />
CA10µg),(Tic),Azlocillin 100µg, Timentin<br />
(Tim) (75µgTic+CA 10 µg), Cefaloridin(Cfr)<br />
30µg ,Cefamandol (Cfm) 30µg and<br />
Ceftazidime (Cfz) 30µg, Cefixime(Cxm)<br />
30μg, Ceftriaxone (Ctr) 30µg, Cefoperazon<br />
(Cfp) 30µg ,Aztreonam (Atm) 30µg<br />
Rifampicin (Rif) 30µg, Nalidixic acid<br />
(Nal)30µg, Ciprofloxacin(Cip) 10mcg,<br />
Amikacin (Amik)10µg (Tc)30µg,<br />
Chloramphenicol (Cm)30µg, Gentamicin<br />
(Gm)30µg, and Cotrimoxazole<br />
(Trimethoprime 2.5 µg + Sulfamethaxazole<br />
22.5 µg) (Tm).<br />
Minimum inhibitory concentrations (MICs)<br />
MICs were determined by dilutions of<br />
different concentrations of Cfm, Cfz, alone<br />
and in the presence of Clavulanic acid (CA).<br />
According to the method recommended by the<br />
National committee for microbiology<br />
Laboratory standards (FRANCE) Powders of<br />
β -lactam antibiotics were obtained from<br />
(Russell and Beecham). (15)<br />
Transfer of genetic information by direct<br />
conjugation method<br />
Conjugal transfer of 3GC resistant ESBL<br />
producing strains was done at 35°C -37°C in<br />
liquid medium {Brain heart infusion (B.H)} or<br />
in solid media {Trypticase Soya agar (T.S.A)<br />
or Mueller – Hinton (M.H)} using E. coli<br />
K12 600 Rif and E.coli K12 600 Nal as<br />
recipient. Equal volumes (1 mL) of culture of<br />
the donor and the recipient strain (108-109<br />
CFU/mL) grown with agitation in tryptic soya<br />
broth were mixed and incubated statically for<br />
18 hours at 35°C. Transconjugants were<br />
selected on M.H agar containing 64-µg/mL<br />
Nalidixic acid to inhibit the growth of donor<br />
and 2.5 µg/mlCfz to inhibit the growth of<br />
recipient strain (11) .
Iraqi J Pharm Sci, Vol.19(2) 2010 Stability of Cfm and Cfz with CA against β- lactamase<br />
Phenotypic confirmatory disc diffusion test<br />
(PCDDT) for ESBL (18)<br />
Ten µl of CA solution was added to discs<br />
of Cfz and Cxm one hour before culture, these<br />
were applied to the surface of a Muller Hinton<br />
agar, seeded with a suspension of 10 4 -<br />
10 5 /CFU of bacteria under test.. An increase in<br />
zone diameter for either antimicrobial agent<br />
tested in combination with CA versus its zone<br />
when tested alone was observed. For Cfz an<br />
increase in zone diameter of> 5mm and for<br />
Cxm > 3 mm was considered as an ESBL<br />
producer.<br />
Extraction of ß – lactamase<br />
Cell free beta –lactamases were prepared<br />
from strains known to be good producers of<br />
the desired enzymes, (ß –lactamases, type<br />
TEM-1 and SHV-1, R-plasmid mediated<br />
enzymes) and ß –-lactamase from E.cloacae<br />
P99 ( cephalospornase) as references. Crud<br />
enzymes also prepared from test isolates of<br />
E.coli (10) .<br />
Detection of ß – lactamase by Macro -<br />
iodometric method.<br />
This test is based on the reaction of the<br />
(oic) acid of penicillin with iodine. ß –<br />
lactamasehydrolyze penicillin to penicilloic<br />
acid, which in turn react with iodine, the<br />
presence of ß – lactamase in a test system was<br />
shown by decolorization of starch-iodine<br />
complex, observed in 1-18hours at 4°C (16) .<br />
Assessment of stability of ß – lactams to cellfree<br />
ß – lactamases (17)<br />
The surface of Muller Hinton agar was seeded<br />
with a suspension of sensitive indicator E.coli<br />
ATCC. Four discs containing ß – lactams<br />
under test were placed near filter papers discs;<br />
each of them was impregnated with 30μl of<br />
the extract enzymatic. The plates were<br />
incubated at 37°C for 18hours, the ß –<br />
lactamase activity was observed like half<br />
moon zone of inhibition.<br />
Masuda microbiological method (17)<br />
Ten clinical isolates were screened for ß –<br />
lactamase inhibitors using 10μl CA in<br />
combination with 30 μl of Cfz or Cfm.<br />
Sensitivity discs containing Cfz or Cfm and a<br />
filter disc incorporated with10μl enzyme and<br />
10μl (CA) as potassium clavulanate were<br />
placed on agar plate on which a bacterial<br />
suspension of sensitive E.coli ATCC<br />
(standard) was spread the inoculum was 10 4 –<br />
10 5 CFU / ml, of 6 hours cultures at 35 C 0 -<br />
37C 0 for 24 hours. Unchangeable inhibition<br />
zones demonstrate stability of the antibiotic to<br />
the enzyme.<br />
44<br />
Results and Discussion<br />
Extended –spectrum ß – lactamases ( ESBLs)<br />
are derivatives of enzymes such as SHV-1 and<br />
TEM-1 that have undergo site specific<br />
mutation that enable them to hydrolyze , and<br />
thus inactivate , oxyimino – cephalosprins<br />
such as, cefotaxime and ceftazidim. (19) . All<br />
clinically important reactions of ß – lactamase<br />
inhibitors , such as tazobactam , sulbactam,<br />
and calvulanic acid , involve ß – lactam ring<br />
cleavage during acylation of an active site .<br />
Although other clavams produced in nature<br />
may possess antibacterial and antifungal<br />
properties, clavulanic acid is the only one<br />
known clavam with potent ß – lactamase<br />
inhibitory activity owing in part to its 3R ,5R<br />
stereochemistry , it is a potent inhibitor of ß –<br />
lactamase enzymes produced by many strains<br />
of Staphylococcus aureus , E.coli , Klebsiella ,<br />
Proteus , Sheglla , Pseudomonas , and<br />
Haemophilus influenzae (20,21,) .<br />
100% of the isolates were found to be resistant<br />
to Amp, Amo,Cb,Tic, Azl, Cfr,Cfo, Tc,Cm<br />
and Tm , 10% were resistant to Cfm Cxm,<br />
Cfz,Cfp,Ctr , Atm Tim, and Amc.Also<br />
resistant to G,Amk,Cip and Tm , 50% of the<br />
isolates were resistant to Nal and Rif as shown<br />
in Table 1. ESBL was detected in 10 isolates<br />
by PCDDT the zone of inhibition increased<br />
in presence of CA. For Cfz >10mm, and for<br />
Cfm and Cxm>5mm, potentiation of the<br />
inhibiton zone of 3GC in the presence of CA<br />
was observed.. indicated ESBL production in<br />
ten strains; the diameters zone of inhibition for<br />
Amc and Tim were range from 0 - 5mm while<br />
the normal diameters zones of inhibition were<br />
for Amc 14-21mm and for Tim is 13mm. The<br />
critical normal MICs for Tim and Amc were<br />
(4-16) and (128) respectively. The MICs were<br />
studied for ten clinical isolates of E.coli in<br />
comparison with standard resistant strains, the<br />
range of MICs for Cfm was 512 - 2048 μg/ml<br />
and for Cfz 32-64 μg/ml, while for non ESBL<br />
producer it ranged from 0.02-8 µg/mL. After<br />
the addition of CA eight-fold reduction or<br />
more in MICs (Table 2 ) . These results was in
Iraqi J Pharm Sci, Vol.19(2) 2010 Stability of Cfm and Cfz with CA against β- lactamase<br />
agreement with the investigation of Chaudhary<br />
,U, Aggarwal-R (17) indicated ESBL producers.<br />
All the isolates were sensitive to Imp, but<br />
among the non ß – lactam antibiotics Cip and<br />
Amk were most effective drugs 90 strains<br />
were sensitive. Resistance to Cfz was<br />
transferred to recipient E. coli K12 C600 Rif or<br />
E. coli K12 C600 Nal strains, along with<br />
resistance to other ß – lactam antibiotics, ESBL<br />
production is coded by genes on conjugation<br />
plasmids which are easily transmitted among<br />
different members of Enterobacteriaceae, all<br />
ESBLs have serine at their active sites. The<br />
results of detection of ß – lactamases by<br />
iodometric method were positive for 10 strains<br />
comparing with standard negative and positive<br />
ß – lactamases R111 (TEM-1) and E.coli<br />
ESBL producer. The inhibition of betalactamase<br />
production by CA has been<br />
NO of<br />
isolates<br />
45<br />
demonstrated with many strains of bacteria,<br />
this effect potentiates the action of many beta<br />
lactams, such as Amp, Amo, Cb and Azl.<br />
Many clinical reports of combination of Amo<br />
with CA have been encouraging, in urinary<br />
tract infections due to ß – lactamase-producing<br />
organisms type TEM and SHV, whilst Amo<br />
alone had no effect, the addition of CA (as<br />
salt) dramatically change the half moon<br />
inhibition zone to complete inhibition zone<br />
(3,4,11) . Figure 1 indicate the activity of ß –<br />
lactamase extracts against ß -lactams<br />
antibiotics, figure 2 indicate the Antibiotic –<br />
enzyme Interaction by the highly sensitive<br />
double disks technique, demonstrated their<br />
hydrolysis, however ß -lactamase of E.cloacae<br />
not effected by Amc and inhibited by Azl and<br />
hydrolyzed all cephalosporins. ( 5,6,20) .<br />
Table 1: Se nsitivity Tests of Ten Strains De termine d by Disk Diffusion Te st .<br />
Diameters of zone of inhibition / MM<br />
Amo Amc Tic Tim Cxt Cfm Cfz Cxm Ctr Amk Cip Rif Nal<br />
1 0 4 0 5.5 16 2 3 10 11 10 12 19 0<br />
2 0 4.5 0 7 16 3 3 12 10 11 10 0 18<br />
3 0 3.5 0 8 18 2 5 8 5 14 12 19 0<br />
4 0 4 0 8.5 17 0 4 4 13 12 11 10 0<br />
5 0 5 0 9 17 6 14 15 12 12 11 19 0<br />
6 0 5.5 0 9 19 10 14 15 13 12 18 0 0<br />
7 0 6 0 7 20 11 11 15 12 18 20 0 18<br />
8 0 6 0 6.5 20 5 10 13 10 18 24 0 19<br />
9 0 6 0 9.5 20 5 11 12 10 15 22 19 19<br />
10 0 7.5 0 9 17 7.5 13 10 10 12 10 19 18<br />
E.coli<br />
(ESBL)<br />
E.coli<br />
0 0 0 5 17 7 13 12 10 12 18 11 17<br />
ATCC<br />
25922<br />
21 21 13 13 22 22 21 21 21 32 40 32 33<br />
Abbreviation's : Amo: Amoxicillin Amc:Amoxiclave; Cb: Carbenicillin;Azl: Azlocillin; Tim: Timentin; Cxt Cefoxitin ; Cfm:<br />
Cefamandol; Cfz : Ceftazidime; Cxm:Cefixime,Ctr:Ceftriaxone, Cfp :Cefoperazon; Amk: Amikacin, Cip: Ciprofloxacin , Rif:<br />
Rifampicin; Nal: Nalidixic acid; the diameters of zone of inhibition for Amp icillin, Amoxic illin, Carbenicillin,Ticarcillin,<br />
Azlocillin,Cefazolin(Cfo), Cefaloridin(Cfr),Cefoperazone(Cfp) Aztreonam(Atm) , Tetracycline ; Chloramphenicol;<br />
Trimethoprim and Gentamicin were zero. All of them sensitive to Imipenem (Imp) 17-23mm and Cefoxitin 15-22mm .Normal<br />
zone for Amc: 14-21mm; Tim:13mm; Cfz,Ctr,Ctx: 15-21mm; Cfm:15-22mm. Amk:25-32mm; Rif :19-32mm;Cip:30-40mm.<br />
Table 2: Minimum Inhibitory Concentrations of Ten Uropathoge nic E.Coli Co mparing with<br />
Standard Strains .<br />
MICs mcg/ml<br />
No . o f Iso late E.coli<br />
Imp Cfm Cfz Cfm+CA Cfz+CA<br />
1,2,3 2 512 64 2 1<br />
4,5,6 1 1024 64 0.5 2<br />
7,8,9,10 4 2048 32 0.25 0.5<br />
E.coli (453)* SHV-1 (7.7) (19) 16 16 0.05 0.25 0.25<br />
E.coli (R111) TEM-1 (5.4)* ) 16 32 0.02 0.25 0.25<br />
E.cloacae(P99** (8.3)* 64 512 64 32 64<br />
E.coli (ESBL) 4 128 64 0.25 2<br />
* Isoelectric points. ** Cephalosporinase.<br />
Normal values of MICs : Cfm S32mcg\ml<br />
Cfz S16mcg\ml
Iraqi J Pharm Sci, Vol.19(2) 2010 Stability of Cfm and Cfz with CA against β- lactamase<br />
Cefoxitin Cefazolin Cefamandol Amoxicillin<br />
Ceftriaxone Azthreonam Ceftazidime Cefixime<br />
Imipenem Cefalo ridin Azlocillin Carbenicillin<br />
Figure 1: Activity of ß – lactamase against ß -<br />
lactams antibiotics<br />
Figure 2: Antibiotic –enzyme Interaction ,by<br />
the highly se nsitive double disks technique (24)<br />
demonstrate d<br />
A : hydrolysis of ceftazidime and Cefalmandol<br />
by β-Iactamases producing E.coli<br />
B: Inhibition by Clavulanic acid (CA) .<br />
Conclusions<br />
The Ten clinical isolates in this study<br />
were very resistant to Amc, Tim,Cfm<br />
,Cfz,Cxm,Cfp, Ctr and Atm, but sensitive to<br />
Imipenem comparing with standard TEM-1<br />
and SHV-1 (plasmidic penicillinases) and<br />
E.clocae P99 ) (Chromosomic<br />
Cephalosporinase) indicating the prevalence of<br />
extended-spectrum β-lactamases (ESBLs)<br />
enzymes that hydrolyze and cause resistance<br />
to oxyimino-cephalosporins and aztreonam.<br />
Our study shows presence of ESBL producer<br />
E.coli in ten clinical isolates. The routine<br />
antimicrobial sensitivity test may fail to detect<br />
ESBL, mediated resistance against 3GC and<br />
detection of ESBL production should be<br />
carried out as a routine in diagnostic<br />
laboratories by PCDDT as it is a simple and<br />
cost effective test, the combination with<br />
Clavulanic acid bringing the susceptibility<br />
back, confirms the ESBLs. ESBLs have<br />
become widespread throughout the world and<br />
are now found in a significant percentage of<br />
E.coli and Klebsiella pneumoniae strains in<br />
certain countries, (6, 7, 9, 10) . The increasing<br />
emergence of cephalosporins resistant E.coli<br />
has leaded to concern about the use of various<br />
combination therapies.<br />
References<br />
1. Jensen, -S-E; Paradkar, -A-S; Mosher, -R-<br />
H; Anders, -C; Beatty, -P-H; Brumlik, -<br />
M-J; Griffin, -A; Barton, -B.Five<br />
additional genes are involved in<br />
clavulanic acid biosynthesis in<br />
Streptomyces clavuligerus.Antimicrob-<br />
Agents-Chemother. 2004; 48(1): 192-202<br />
2. Dumon L., Adriaens P., Anne J., Eyssen<br />
H. Effect of Clavulanic acid on the<br />
minimum inhibitory concentrations of<br />
benzylpenicillin, ampicillin, carbenicillin,<br />
or cefalothin against clinical isolates<br />
resistant to beta-lactam antibiotics.<br />
Antimicrob. Agents and Chemother.,<br />
1979,N2,315-317<br />
3. Bush, K., Jacoby,G.,A and Medeiros.A.,A<br />
. A functional classification scheme for<br />
beta – lactamases and its correlation with<br />
molecular structure.Antimicrobial agents<br />
chemotherapy 1995 p1211-1233.<br />
4. Poeschl, -P-W; Eckel,-D; Poeschl,-E. Post<br />
operative prophylactic antibiotic<br />
treatment in third molar surgery--a<br />
necessity? J-Oral-Maxillofac-Surg. 2004;<br />
62(1): 3-8; discussion 9IS<br />
5. Bradford, -P-A. Extended-spectrum βlactamases<br />
in the 21 st century:<br />
characterization, epidemiology, and<br />
detection of this important resistance
Iraqi J Pharm Sci, Vol.19(2) 2010 Stability of Cfm and Cfz with CA against β- lactamase<br />
threat.Clin-Microbiol-Rev. 2001; 4(4)<br />
933-51.<br />
6. Sturenburg, -E; Mack, -D.Extendedspectrum<br />
beta-lactamases: implications<br />
for the clinical microbiology laboratory,<br />
therapy, and infection control. J-Infect.<br />
2003; 47(4): 273-95<br />
7. Bell,-J-M;Turnidge,J-D;Jones,R-N.<br />
Prevalence of extended-spectrum betalactamase-producing<br />
Enterobacter cloacae<br />
in the Asia-Pacific region: results from<br />
the sentry Antimicrobial Surveillance<br />
Program, 1998 to 2001. Antimicrob-<br />
Agents-Chemother.2003; 47(12): 3989-93<br />
8. Edelstein, -M; Pimkin, -M; Palagin, -I;<br />
Edelstein,-I; Stratchounski,-L .Prevalence<br />
and molecular epidemiology of CTX-M<br />
extended-spectrum beta – lactamase -<br />
producing E.coli and Klebsiella<br />
pneumoniae in Russian hospitals.<br />
Antimicrob- Agents- Chemother. 2003;<br />
47(12): 3724-32<br />
9. Bauernfeind, A., H. Grimm, and<br />
S.Schweighart. A new – plasmidic<br />
cefotaximase in clinical isolate of E.coli.<br />
Infection. 1990 18: 294-298 .<br />
10. Siham., S.S, Joly,B, . Phillipon ,A.<br />
,Sirot,D.,Cluzel.R. Resistance al’a<br />
carbenicilline des bacilles a Gram-<br />
negatife , frequance, determinisme<br />
biochemique et genetique, 1985 Pathol<br />
,Biol., 33, 825-829.<br />
11. El-Sukhon,-S-N; Faiza-Boukhatem,-Z.<br />
Activity of combinations of ceftazidime,<br />
imipenem and pefloxacin against<br />
Staphylococcus aureus, E. coli and P.<br />
aeruginosa. Int-J-Antimicrob-Agents.<br />
2003 Dec; 22(6): 613-7.<br />
12. Kamidono, S et al. A comparative study<br />
on the clinical utility of cefozopran and<br />
cefpirome against complicated urinary<br />
tract infections. Jap.J. Antibiot.2000;<br />
53(6): 430-450.<br />
13. Dromigny, -J-A; Ndoye, -B; Macondo, -<br />
E-A; Nabeth, -P; Siby, -T; Perrier-Gros-<br />
Claude, -J-D.Increasing prevalence of<br />
antimicrobial resistance among<br />
47<br />
Enterobacteriaceae uropathogens in<br />
Dakar, Senegal: a multicenter study.<br />
Diagn-Microbiol-Infect-Dis. 2003; 47(4):<br />
595-600.<br />
14. Cowan, S.T, Manual for identification of<br />
medical bacteria 1977. P106-Cambridge<br />
university press. Cambridge New York.<br />
15. Baur A. W., Kirby, W. M.M., Sherris<br />
K.C. and Turck, M.Antibiotic<br />
susceptibility testing by a standardized<br />
single disc method.,Amer. J. clin.path.<br />
1966, 45,493-496<br />
16. Labia, R., Barthelemy.M. L enzymogram<br />
des ß -lactamases .A daptation en gel .La<br />
method iodometrique, Ann. Macrobiol.,<br />
1979, 130B, 236-240.<br />
17. Masuda G., Tomioka s and Haregawa M.,<br />
Detection of ß -lactamases production by<br />
Gram-negative bacteria.The journal of<br />
Antibiotics, 1976, 29(6),662-664<br />
18. Chaudhary,U,Aggarwal-R. Extendedspectrum<br />
beta-lactamases.An emerging<br />
threat to clinical therapeutics.Indian J-<br />
IMed. Microbiol2004; 22(2): 75-80<br />
19. Matthew Kalp et al. Why extended –<br />
spectrum ß – lactamases SHV-2 and SHV-<br />
5 are ' Hypersuceptible' to Mechanism<br />
based Inhibitors, Biochemistry , 2009, 48<br />
(41) , 9912-9920<br />
20. Mattew Kalp et al . Efficient Inhibition of<br />
Class A and Class D ß – lactamases by<br />
Michaelis Complexes , Journal of<br />
Biological Chemistry , 2007.28(30) .<br />
21. Mary L. Raber , Micheal F. Freeman and<br />
Criag A.Twonsend , Journal of Biological<br />
Chemistry , 2009, 284 (1).<br />
22. Siham-SS. Marc O. Sirot-D- Joly-B. and<br />
Cluzel –R Spread of SHV-1 betalactamases<br />
in E.coli isolated from fecal<br />
samples in Africa.1987. Antimicro. Agent<br />
and Chemother.vol 31 (6)943-945.<br />
23. -Ma, -Y; Li, -J-Y; Yao, -L; Zhang, -L;<br />
Hu,-C-Q; Jin,-S-H Zhonghua-Yi-Xue-<br />
Za-Zhi. Antimicrobial resistance of<br />
Escherichia coli isolates collected from<br />
inpatients and outpatients] 2003 25;<br />
83(12): 1046-8.
Iraqi J Pharm Sci, Vol.19(2) 2010 Gravimetric estimation of caffeine<br />
Gravimetric Estimation of Caffeine in Different Commercial Kinds<br />
of Tea Found in the Iraqi Market<br />
Maha N. Hamad* ,1 and Dhuha A. Abdul-Hussain*<br />
*Department of Pharmacognosy, College of Pharmacy, University of Baghdad, Baghdad,Iraq .<br />
Abstract<br />
Caffeine (1,3,7-trimethylxanthine), which is the most widely consumed stimulant in the world,<br />
had been isolated and estimated gravimetrically in fifteen different commercial kinds of tea found in<br />
the Iraqi market.The kinds of tea were chosen according to their differences in the degree of<br />
fermentation and the method of processing i.e. black , gray and green . The isolated caffeine was<br />
identified by melting point, sublimation, TLC, chemical tests, UV , IR , HPLC and CHNO analysis.<br />
Key words: Caffeine , Purine, te a.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﺔﻘﻳﺮﻄﺑ ﻪﺘﻴﻤﻛ ﻦﻴﻴﻌﺗﻭ ﻪﻠﺼﻓ ﻢﺗ , ﻢﻟﺎﻌﻟﺍ<br />
ﻲﻓ ًﻻﺎﻤﻌﺘﺳﺇ ﺔﻬﺒﻨﻤﻟﺍ ﺩﺍﻮﻤﻟﺍ ﺮﺜﻛﺃ ﻦﻣ ﺮﺒﺘﻌﻳ ﻱﺬﻟﺍ ( ﻦﻴﺜﻧﺍﺯ ﻞﻴﺜﻣ ﻲﺛﻼﺛ – 7 , 3 , 1)<br />
ﻦﻴﻳﺎﻓﺎﻛ<br />
ﺮﻴﻤﺨﺘﻟﺍ ﻦﻣ ﺔﻔﻠﺘﺨﻣ ﺕﺎﺟﺭﺩ ﻰﻠﻋ ﻱﺎﺸﻟﺍ ﻦﻣ ﺝﺫﺎﻤﻧ ﺭﺎﻴﺘﺧﺃ ﻢﺗ . ﺔﻴﻠﺤﻤﻟﺍ ﻕﺍﻮﺳﻷﺍ ﻲﻓ ﺩﻮﺟﻮﻤﻟﺍ ﻱﺎﺸﻟﺍ ﻦﻣ ًﺎﻔﻠﺘﺨﻣ ًﺎﻋﻮﻧ ﺮﺸﻋ ﺔﺴﻤﺧ ﻲﻓ ﺔﻴﻧﺯﻭ<br />
, ﻥﺎﺑﻭﺬﻟﺍ ﺔﺟﺭﺩ ﺱﺎﻴﻗ ﺎﻬﻨﻣ ﺔﻔﻠﺘﺨﻣ ﻕﺮﻄﺑ ﻝﻭﺰﻌﻤﻟﺍ ﻦﻴﻳﺎﻓﺎﻜﻟﺍ ﺺﻴﺨﺸﺗ ﻢﺗ . ﺮﻀﺧﻻﺍﻭ ﻲﺻﺎﺻﺮﻟﺍﻭ ﺩﻮﺳﻻﺍ ﻱﺍ ﺮﻴﻀﺤﺘﻠﻟ ﺔﻔﻠﺘﺨﻣ ﻕﺮﻃ ﻭ<br />
ءﺍﺮﻤﺤﻟﺍ ﺖﺤﺗ ﺔﻌﺷﻷﺍﻭ ﺔﻴﺠﺴﻔﻨﺒﻟﺍ ﻕﻮﻓ ﺔﻌﺷﻷﺍ ﻒﻴﻃﻭ , ﺔﻳﻭﺎﻴﻤﻴﻛ ﺕﺎﺻﻮﺤﻓﻭ , ﺔﻘﻴﻗﺮﻟﺍ ﺔﻘﺒﻄﻟﺍ ﺎﻴﻓﺍﺮﻏﻮﺗﺎﻣﻭﺮﻛﻭ , ﻲﻣﺎﺴﺘﻟﺍ<br />
. ﻦﻴﺠﺴﻛﻭﻻﺍﻭ ﻦﻴﺟﻭﺮﺘﻴﻨﻟﺍ , ﻦﻴﺟﻭﺭﺪﻴﻬﻟﺍ , ﻥﻮﺑﺮﻜﻟﺍ ﺮﺻﺎﻨﻋ ﺐﺴﻧ ﺏﺎﺴﺣﻭ ﺔﻴﻟﺎﻌﻟﺍ ﺓءﺎﻔﻜﻟﺍ ﺕﺍﺫ ﺔﻠﺋﺎﺴﻟﺍ ﺎﻴﻓﺍﺮﻏﻮﺗﺎﻣﻭﺮﻜﻟﺍﻭ<br />
Introduction<br />
Thea or tea consists of the prepared<br />
leaves and leaf buds of camellia sinensis<br />
(formerly known as Thea sinensis) of the<br />
Theaceae family. There are three main<br />
commercial types of tea: green , oolong (gray)<br />
and black, depending on the method of<br />
processing. The leaves may be fermented or<br />
left unfermented. Fermented teas are referred<br />
to black tea, unfermented teas as green tea and<br />
partially fermented teas as oolong tea.Black tea<br />
is prepared by heaping the fresh leaves until<br />
fermentation has begun, then they are rapidly<br />
dried artificially with heat, while green tea is<br />
prepared by rapidly drying the freshly picked<br />
leaves in copper pans over a mild artificial<br />
heat, or the leaves are often rolled in the palm<br />
of the hand as they dry. Gray tea is partially<br />
fermented by heaping then they are dried on<br />
N<br />
N<br />
N<br />
H<br />
N<br />
O<br />
NH<br />
O<br />
H<br />
N<br />
48<br />
artificial heat. Tea contains caffeine (theine)<br />
and small amounts of adenine, theobromine,<br />
theophylline, gallotannic acid and volatile oil.<br />
1,2 Caffeine (1,3,7-trimethylxanthine), the<br />
molecular formula of which is C8H10N4O2 , is<br />
the most widely consumed stimulant in the<br />
world can be considered to be constructed<br />
from the purine ring system, which is<br />
important biologically, being found in nucleic<br />
acids and nucleotide and in few organisms as<br />
alkaloids. 3 Caffeine was first discovered in tea<br />
in 1827, and was named theine , and later it<br />
was found in mate , coffee and various other<br />
plants and the term theine was then<br />
dropped. 4 Purines are considered pseudo<br />
alkaloids since they are not derived from an<br />
amino acid precursor. 5<br />
N<br />
H<br />
N<br />
H 3C-N<br />
Purine X anthine C affeine<br />
1Corresponding author E- mail : manoha_1957@yahoo.com<br />
Received : 28/4/2010<br />
Accepted : 8/8/2010<br />
O<br />
O<br />
N<br />
CH 3<br />
N<br />
N<br />
CH 3
Iraqi J Pharm Sci, Vol.19(2) 2010 Gravimetric estimation of caffeine<br />
Caffeine acts as a CNS stimulant , mild<br />
diuretic, it increases the heart rate and blood<br />
pressure and stimulate gastric secretions. It<br />
also acts as a natural pesticide that paralyze<br />
and kills certain insects feeding on the plants.<br />
6,7 Caffeine with UV can kill some kinds of<br />
algae and there are evidences that it enhances<br />
mutations in bacteria and viruses and also<br />
induce chromosome damage. 8,9,10,11 Caffeine is<br />
an ingredient of several dozen proprietary<br />
products, for the most part, these combination<br />
with acetyl salicylic acid, ascorbic acid,<br />
codeine, paracetamol, and other analgesics<br />
and antipyretics.Caffeine is found in a number<br />
of beverages ingested by people in addition to<br />
tea as coffee, and to some extent cocoa. Other ,<br />
less commonly used sources of caffeine<br />
include the plants guarana, and yerba mate'<br />
which are sometimes used in the preparation of<br />
teas, and recently ,energy drinks. Tea leaves<br />
contain 1-4% caffeine, while coffee 1-2% yet a<br />
cup of brewed coffee contains about 100-150<br />
mg caffeine while a cup of tea contains 60-75<br />
mg. caffeine is also a common ingredient of<br />
soft drinks such as cola. Soft drinks typically<br />
contain about 10-50 mg of caffeine per serving<br />
. The range of caffeine contents in various<br />
beverages is shown table 1<br />
Table 1 : range of caffeine in various<br />
beve rages<br />
Approximate caffe ine<br />
content of various<br />
beverages<br />
Coffee (5oz. cup)<br />
Range of mgs<br />
of caffeine<br />
40-170<br />
Soft drinks (12 oz. can) 10-50<br />
Black tea (one tea bag) 25-110<br />
Oolong tea (one tea bag) 12-55<br />
Green tea (one tea bag) 8-30<br />
Decaffeinized tea(one tea<br />
bag)<br />
1-4<br />
Energy drinks ( 12 0z. can) 75-90<br />
Diagram 1 : Isolation of caffe ine from te a<br />
49<br />
In this paper we have estimated caffeine<br />
gravimetrically in fifteen different kind of tea<br />
found in the market black, gray and green tea .<br />
Materials and method<br />
Samples of tea were chosen randomly to<br />
represent black, gray and green tea in the<br />
form of tea bags or unpacked form.<br />
All reagents are anhydrous solvents were of<br />
analar type and generally used as received<br />
from the commercial suppliers.<br />
Silica gel used in the form or ready made<br />
aluminum plates of silica gel GF254 , Merck<br />
Co.<br />
UV was run in methanol , IR was run in KBr<br />
disk.<br />
HPLC was done using Knauer/ Germany<br />
HPLC.<br />
Standard caffeine is from Evans Medical Ltd<br />
, Liverpool.<br />
Isolation of caffeine<br />
25 gm of tea were boiled with 200ml of<br />
water for fifteen minutes in a covered beaker .<br />
The extract was filtered through muslin and the<br />
mark was re boiled with 120ml of water for<br />
five minutes, filtered and the mark over the<br />
muslin was washed with 70ml of boiling water,<br />
the muslin was then squeezed till exhaustion.<br />
The combined extracts were cooled and mixed<br />
with 4gm of sodium carbonate with stirring,<br />
then transferred to a separatery funnel and<br />
partitioned with three successive portions of<br />
methylene chloride each of 50ml (i.e.<br />
3X50ml), each time the funnel was inverted<br />
back and forth ten times. The methylene<br />
chloride layers were combined together and<br />
dried over anhydrous sodium sulfate , filtered<br />
and evaporated to dryness under vacuum. The<br />
obtained caffeine was re crystallized from<br />
boiling ethanol, filtered and weighed. The<br />
percentages of caffeine was calculated as w/w.<br />
The extraction procedure is shown in<br />
diagram(1).
Iraqi J Pharm Sci, Vol.19(2) 2010 Gravimetric estimation of caffeine<br />
Two samples of each kind were used and the<br />
average weights of the isolated caffeine were<br />
taken for calculation of the percentages and<br />
comparison.<br />
Identification of Caffeine<br />
The isolated caffeine was identified by<br />
several methods :<br />
It was identified by measuring its melting<br />
point and compare it with standard caffeine<br />
and also using a mixed sample from isolated<br />
caffeine and the standard . 12 The results are<br />
shown in table (2).<br />
Table 2 : Melting points of isolated and<br />
standard caffe ine<br />
Sample<br />
Isolated caffeine<br />
Standard caffeine<br />
Mixed isolated and<br />
Standard caffeine<br />
Melting point<br />
236.7°C<br />
237.3°C<br />
237°C<br />
Then caffeine was identified by two chemical<br />
tests :<br />
1- Murexide test : Isolated caffeine gave a<br />
purple color.<br />
2- Isolated caffeine was treated with hydrogen<br />
peroxide and 2% HCL. After evaporation<br />
to dryness , a bright red color was obtained.<br />
The color turn purple upon addition of<br />
drops of 5% ammonia. 13<br />
Also caffeine was identified by sublimation<br />
and this process was achieved by introducing a<br />
small quantity of caffeine in a porsalen dishand<br />
covered with a watch glass , the porsalen dish<br />
was subjected to heat while the watch glass<br />
was covered with a plastic sack containing ice<br />
.Upon heating caffeine started to sublime and<br />
condense on the lower surface of the watch<br />
glass. Caffeine was also identified by TLC<br />
using silica gel GF254 plates developed in three<br />
different mobile phases and comparing the Rf<br />
Figure 2 : IR spectrum of the isolate d caffeine<br />
50<br />
values of isolated caffeine with standard using<br />
single and mixed spots , and detection was<br />
done under UV254nm. 14,15,16,17<br />
Mobile phases used are:<br />
Mobile phase I : Ethyl acetate : acetic acid<br />
95:5 .<br />
Mobile phase II : Chloroform : ethyl acetate :<br />
formic acid 5: 4 : 1<br />
Mobile phase III : petroleum ether : methylene<br />
chloride : ethyl acetate 1: 1 : 2.<br />
The result of TLC are shown in table (3)<br />
Table 3 : Rf value s of isolate d and standard<br />
caffeine<br />
Mobile<br />
phase<br />
Isolated<br />
caffe ine<br />
Rf value<br />
Standard<br />
Caffeine Rf<br />
values<br />
I<br />
0.226 0.257<br />
II 0.490 0.516<br />
III 0.110 0.110<br />
The UV absorption spectrum exhibits a pair<br />
of absorption bands peaking at (209) and<br />
(272)nm with a shoulder between them 18 .<br />
The UV spectrum of the isolated caffeine is<br />
shown in fig.(1)<br />
Figure 1 UV spe ctrum of the isolated<br />
caffeine<br />
Also caffeine was identified by IR 19 and the<br />
spectrum is shown in fig. (2)
Iraqi J Pharm Sci, Vol.19(2) 2010 Gravimetric estimation of caffeine<br />
Also caffeine was identified by CHNO<br />
analysis ,whereby the percentage of each<br />
element measured and compared with the<br />
calculated one.The results are shown in table 4.<br />
Table 4 : CHNO analysis of caffeine<br />
Ele ment Calculate d Found<br />
perce ntage s pe rcentages<br />
Carbon 49.484 49.877<br />
Hydrogen 5.155 5.199<br />
Nitrogen 28.866 29.109<br />
Oxygen 16.495 16.709<br />
Figure 3 : HPLC of isolated caffeine<br />
Figure 4 : HPLC of standard<br />
Results and Discussion<br />
The average weights and percentages of the<br />
caffeine isolated from each kind of tea are<br />
shown in table (5) and fig. (5). The idea of this<br />
method of isolation of caffeine is to extract the<br />
water soluble materials in the tea leaves in a<br />
hot water . (the solubility of caffeine in water<br />
is 22 mg/ml at 25 o c , 180 mg/ml at 80 o c and<br />
760 mg/ml at 100 o c ) . The caffeine is<br />
extracted from the water after cooling with<br />
dichloromethane (140 mg/ml) than in water<br />
(22 mg/ml), it readily dissolves in the<br />
51<br />
Caffeine was finally identified by HPLC 18<br />
using C18 5x150mm column and a mobile<br />
phase composed of methanol/water 90:10 with<br />
flow rate of 0.8ml/minute and detection with<br />
UV detector at 275nm. The retention time of<br />
the isolated caffeine was compared with that of<br />
the standard. The results are shown in figures 3<br />
and 4.<br />
dichloromethane.However, the tannins are<br />
slightly soluble in dichloromethane but upon<br />
addition of sodium carbonate to the extract the<br />
tannins will be converted to phenolic anions<br />
(since phenols are acidic enough to be<br />
converted to phenolic salts i.e. deprotenation of<br />
OH group ) upon addition of sodium<br />
carbonate). The phenolic salts thus formed are<br />
not soluble in dichloromethane , soluble in<br />
water, as shown below:<br />
ArOH + Na + 2CO3 -2 → ArO - Na + + Na + HCO3 -<br />
tannins soluble tannins salts<br />
in water, dichloromethane soluble in water<br />
insoluble in dichloromethane
Iraqi J Pharm Sci, Vol.19(2) 2010 Gravimetric estimation of caffeine<br />
Table 5 :The perce ntage of caffe ine in differe nt kinds of te a<br />
no<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
7<br />
8<br />
9<br />
10<br />
11<br />
12<br />
13<br />
14<br />
15<br />
% Percentage of cafe in<br />
2.5<br />
Tea brand<br />
Al-Otoor (black)<br />
Al-Rabeea (black)<br />
Mahmood (black)<br />
Ahmad (black )<br />
Al-Wazza (black )<br />
Al-Tuffaha (black )<br />
Ahmad (black , Tea bags )<br />
Ration tea ( black )<br />
Lipton (black , tea bags )<br />
Al-Okozay ( gray , tea bags )<br />
Al-attar ( green , tea bags )<br />
Lipton (green , tea bags )<br />
Ahmad (green , tea bags )<br />
Green tea (un packed)<br />
Alokozay ( green , tea bags )<br />
2<br />
1.5<br />
1<br />
0.5<br />
0<br />
Figure 5 : percentages of caffeine in diffe rent kinds of tea<br />
Caffeine being a xanthine derivative was first<br />
identified by the murexide test. The core of the<br />
reaction is apparently that caffeine through a<br />
number of steps , is oxidized into the<br />
intermediate that ultimately condenses to<br />
murexoin. The ammonium salt of murexoin is<br />
the principal contributor to the purple color of<br />
the final solution.<br />
20 The quantitative<br />
differences obtained in different kind of tea is<br />
probably due to the method of processing of<br />
each kind since caffeine sublimes with out<br />
decomposition upon exposure to heat,<br />
therefore it should be expected that caffeine<br />
could be lost during fermentation and<br />
processing. Also the differences of caffeine<br />
quantity and consequently the percentage may<br />
be due to different time of harvesting of leaves<br />
of the plant.<br />
52<br />
Weight of<br />
caffeine<br />
In 25 gm of tea<br />
0.481<br />
0.351<br />
0.322<br />
0.310<br />
0.294<br />
0.238<br />
0.2<br />
0.180<br />
0.090<br />
0.110<br />
0.341<br />
0.327<br />
0.221<br />
0.130<br />
0.120<br />
% of caffe ine<br />
1.924<br />
1.40<br />
1.288<br />
1.240<br />
1.176<br />
0.952<br />
0.800<br />
0.720<br />
0.360<br />
0.440<br />
1. 364<br />
1.308<br />
0.884<br />
0.520<br />
0.480<br />
Acknowledgement<br />
We are deeply greatfull to Mr. Dhulfiqar<br />
A. Abid ( MSc.) and Mrs. Sahar M. Shakir (<br />
MSc. ) and Mr. Zaid M. Abdul-Khalik for<br />
running the UV , IR , and HPLC spectrum.<br />
References<br />
1. Tyler V.E. , Brady L.E., "Pharmacognosy"<br />
9 th edition, Lea and Febiger Philadelphia<br />
1988.<br />
2. Trease G.E. and Evans W.C.,<br />
"Pharmacognosy" 15 th edition , WB<br />
Saunders Co. Ltd London 2002.<br />
3. Dey P.M. , Harborne J.B. , "Plant<br />
Biochemistry" Academic press 1997.<br />
4. Weinberg BA., Bealer BK., "The world of<br />
caffeine" Routledge, Newyork and<br />
London 2001.
Iraqi J Pharm Sci, Vol.19(2) 2010 Gravimetric estimation of caffeine<br />
5. Cordell G.A., "Introduction to alkaloids"<br />
John Wiley and Sons , Inc Canada 1981, 6.<br />
6. Jinno, D. , "Comperhensive Medical<br />
chemistry" Pergamon Press 1996.<br />
7. Eaton K., "Caffeine could be healpful" Las<br />
Vegas Review Journal , 2010, 23.<br />
8. Srivastava B.S., Kumar H.D., Singh H.N. ,<br />
"The effect of caffeine and light on killing<br />
of the blue-green algae Anabaena doliolum<br />
by UV radiation" Archives of<br />
Microbiology 1971, 78(2), 139-144.<br />
9. Siderpoulos A.S., Shankel D.M.,<br />
"Mechanism of caffeine enhancement of<br />
mutations induced by sub lethal UV<br />
dosages" J. Bacteriology 1968, 96(1), 198-<br />
204.<br />
10. Lytle C.D., "The effect of caffeine on the<br />
survival of UV-irradiated Herpes simplex<br />
Type 1 virus" J. General virology 1974, 24,<br />
381-383.<br />
11. Cremer C., Cremer T., and Gray J. W.,<br />
"Introduction of chromosome damage by<br />
UV light and caffeine" Cytometry 1982,<br />
2(5), 287-290.<br />
12. The Merck index , 8 th edition Merck and<br />
Co. Inc. Rahway, NJ, USA 1996.<br />
13. Cave' A., "Pharmacognosy ,<br />
Phytochemistry, medicinal plants" 3 rd<br />
53<br />
edition , Intercept Ltd, England 1995, 882-<br />
883.<br />
14. Fenske M., "caffeine determination in<br />
human saliva and urine by TLC andUV<br />
absorption densitometry"<br />
Chromatographia 2007, 65(3-4) , 233-238.<br />
15. Pavlik J.W., "The detection of caffeine in<br />
commercial products" J. of Chemical<br />
education 1973, 50(2), 134.<br />
16. Franciszek B., Sabina A., Urszula H.,<br />
"Densitometric determination of caffeine in<br />
tea beverages after TLC e-separation"<br />
Chemia analityczna, 2006, 51(4), 603-611.<br />
17. Stahl, E., "Thin layer chromatography"<br />
Springer-Verlag Berlin. Heidlberg N.Y.<br />
1969.<br />
18. Pavlova V., Petrovsk S., "Simultaneous<br />
determination of amphetamine,<br />
methamphetamine, and caffeine in seized<br />
tablets and HPLC" Acta<br />
Chromatographica, 2007, 18, 157-164.<br />
19. Cook D., Regnier Z.R., "The infrared<br />
spectra of caffeine salts" Canadian Journal<br />
of Chemistry, 1967, 45, 2895-2897.<br />
20. Pedersen O., "Pharmaceutical chemical<br />
analysis : Methods for identification and<br />
limit tests" 2006, 86-88.
Iraqi J Pharm Sci, Vol.19(2) 2010 Goserlin in the management of fibroid<br />
Goserelin versus Norethisterone in the Management of<br />
Menorrhagia with Uterine Fibroid<br />
Faris A. Rasheed * ,1 , Jwan N. Sulaiman* and Yousif Abdul-Raheem<br />
* Departement of Obs/Gyn, Al-Kindy Medical College, University of Baghdad, Baghdad, Iraq.<br />
Abstract<br />
Menorrhagia is common in patients with uterine fibroids, if operation needs to be delayed for a<br />
particular reason, goserelin can be used safely to reduce bleeding and the size of the tumor.The<br />
objective is to compare between goserelin acetate and norethisterone on patients with menorrhagia and<br />
uterine fibroid. A randomized controlled study conducted in Elwiya maternity teaching hospital,<br />
Baghdad from the first of November 2007 to the end of April 2009. 90 patients from the consultant<br />
outpatient clinic with menorrhagia and fibroid, and their operations were delayed for medical reason<br />
were allocated in two groups, the first group, was given 3.2 mg goserelin acetate subcutaneously<br />
monthly for 3 months and the second group was given 5 mg norethisterone orally three times daily<br />
during the attack of bleeding and 5 mg once daily, cyclically if no bleeding for 3 months. The fibroid<br />
was measured in two dimensions, using convex real-time ultrasound before treatment and three months<br />
after treatment. Haemoglobin and the number of pads used were also reported before and after<br />
treatment, also the side effects in both groups and the need for operations.The size of fibroid in two<br />
dimensions measurement was reduced from 28.24 cm 2 ± 6.14 to 12.3 cm 2 ± 3.45 in the goserelin group<br />
(P=0.0001) versus 26.56 cm 2 ± 5.96 to 25.22 cm 2 ± 5.01 in the norethisterone group (P= 0.2589). The<br />
haemoglobin level was 9.28 gm/100ml ± 2.44 pre-treatment in the goserelin group and 11.2 gm/100ml<br />
± 1.88 post-treatment (P= 0.0001) versus 10.08 gm/100ml ± 2.86, and 10.24 gm/100ml ± 2.46<br />
respectively in the norethisterone group (P= 0.7798). The need for operation was decreased significantly<br />
in the goserelin group. Goserelin showed better patient response and reduction in the tumor size than<br />
norethisterone in treatment of patients with menorrhagia and uterine fibroids if operation is delayed for<br />
medical or other reasons.<br />
Key words: Goserelin, Nore thiste rone , Menorrhagia, Uterine Fibroid<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﺺﻴﻠﻘﺗﻭ ﺔﺠﻟﺎﻌﻣ ﻲﻓ Norethisterone ( ﻥﻭﺮﻴﺘﺳ ﻲﺛﺍﺭﻮﻧ ) ءﺍﻭﺩ ﻦﻣ ﻞﻀﻓﺃ ﺞﺋﺎﺘﻧ ﺮﻬﻈﻳ ( Goserlin ) ﻦﻴﻟﺭﺯﻮﻛ ءﺍﻭﺩ ﻥﺇ<br />
ﻰﻟﺇ ﻱﺩﺆﺗ ﺔﻴﺒﻃ ﺏﺎﺒﺳﺃ ﻙﺎﻨﻫ ﻭﺃ ﺔﻳﺮﻬﺸﻟﺍ ﺓﺭﻭﺪﻟﺍ ءﺎﻨﺛﺃ ﺪﻳﺪﺷ ﻑﺰﻧ ﻦﻣ ﻦﻴﻧﺎﻌﻳ ﻲﺗﺍﻮﻠﻟﺍ ﺕﺎﻀﻳﺮﻤﻟﺍ ﺪﻨﻋ ﺔﺻﺎﺧ ﻭ ﻢﺣﺮﻟﺍ ﻲﻓ ﺔﻴﻔﻴﻠﻟﺍ ﺪﻘﻌﻟﺍ ﻢﺠﺣ<br />
ﺔـﺠﻟﺎﻌﻣ ﻲـﻓ ﻝﺎـﻌﻓ ﺮﻬـﺷﺃ 3 ﺓﺪـﻤﻟ ( Goserlin ) ( ﻦﻴﻟﺭﺯﻮـﻜﻟﺍ ) ءﺍﻭﺩ ءﺎـﻄﻋﺇ<br />
ﻥﺃ ﺪـﺟﻭﻭ . ًﺎـﻴﺣﺍﺮﺟ ﺔـﻴﻔﻴﻠﻟﺍ ﺪـﻘﻌﻟﺍ ﻊـﻓﺭ ﺔـﻴﻠﻤﻋ ﻞـﻴﺟﺄﺗ<br />
ﻊﻓﺮﻳﻭ ﻑﺰﻨﻟﺍ ﺓﺪﺷ ﻦﻣ ﻞﻠﻘﻳ ﻪﻧﺍ ﺪﺟﻭ ﺫﺇ ﻢﺣﺮﻟﺍ ﻰﻠﻋ ﺔﻴﻔﻴﻟ ﺪﻘﻋ ﺩﻮﺟﻭ ﺐﺒﺴﺑ ﺔﻳﺮﻬﺸﻟﺍ ﺓﺭﻭﺪﻟﺍ ءﺎﻨﺛﺃ ﺪﻳﺪﺷ ﻑﺰﻧ ﻦﻣ ﻦﻴﻧﺎﻌﻳ ﻲﺗﺍﻮﻠﻟﺍ ﺕﺎﻀﻳﺮﻤﻟﺍ<br />
. ﺔﻔﻴﻔﻃ ﺔﻴﺒﻧﺎﺟ ﺽﺍﺮﻋﺇ ﺩﻮﺟﻭ ﻊﻣ ﻲﺣﺍﺮﺠﻟﺍ ﻞﺧﺍﺪﺘﻟﺍ ﻰﻟﺇ ءﻮﺠﻠﻟﺍ ﻭﺃ ﻡﺩ ءﺎﻄﻋﺇ ﻰﻟﺇ ﺔﺟﺎﺤﻟﺍ ﻞﻠﻘﻳ ﺎﻤﻣ ﻡﺪﻟﺎﺑ ﻦﻴﺑﻮﻠﻛﻮﻤﻴﻬﻟﺍ ﺔﺒﺴﻧ ﻦﻣ<br />
Introduction<br />
Uterine fibroids are the most common<br />
tumor in the female reproductive system. They<br />
are estimated to occur in 25% of women of<br />
reproductive age. (1) In the USA, 30% of women<br />
will have had a hysterectomy by the age of 60<br />
years and 60% will be performed to treat<br />
(2)<br />
fibroids. Hysterectomy or myomectomy<br />
remain the most common types of treatment<br />
and it is associated with high level of<br />
satisfaction. Myomectomy is carried out when<br />
fertility is to be preserved, it can relief<br />
symptoms associated with myoma. Goserelin<br />
acetate, a Gonadotrophin releasing hormone<br />
(GnRH) agonist is a synthetic form of<br />
gonaderelin. It acts on the luteinizing hormone<br />
releasing hormone (LHRH) receptors in the<br />
pituitary gland, in the same way as natural<br />
gonadorelin. The available data seems to<br />
1Corresponding author E- mail : faris_54@yahoo.com<br />
54@yahoo.com<br />
Received : 19/4/2010<br />
Accepted : 18/8/2010<br />
54<br />
support the use of GnRH agonist treatment<br />
before surgery for uterine fibroids in selected<br />
circumstances. (3) Administration of GnRH<br />
agonist for only two or three months<br />
preoperatively in cases of uterine fibroid<br />
decreases the bleeding, mucus debris and<br />
diameter, limiting side effects and cost, (3) and<br />
increase the haematocrit value with no<br />
metabolic side effects or detectable bone<br />
demineralization .<br />
(4) Norethisterne is a<br />
synthetic progestin. It has several indications in<br />
gynaecology and primary care. At low dose ≤<br />
1mg it can be used in contraception and<br />
hormone replacement therapy. At higher dose ≥<br />
5mg it can be used in menorrhagia. (5) In our<br />
study we compare the effect of goserelin<br />
acetate versus norethisterone on patients with<br />
menorrhagia and uterine fibroid.
Iraqi J Pharm Sci, Vol.19(2) 2010 Goserlin in the management of fibroid<br />
Patients and Methods<br />
This study is a randomized controlled<br />
study conducted in Elwiya maternity teaching<br />
hospital, Baghdad from the 1 st of November<br />
2007 to the 30 th of April 2009. The patients<br />
enrolled in the study were 102 women, with<br />
menorrhagia and the presence of uterine<br />
fibroid(s). Patients with pervious myomectomy<br />
and those with known or suspected to have<br />
breast carcinoma were excluded from the<br />
study.All patients were not suitable for near<br />
surgery because of medical problem, long<br />
waiting lists or refusal of surgery by the patient.<br />
Uterine bleeding was considered abnormal<br />
according to the patients subjective evaluation<br />
in comparison with previous menstrual status.<br />
The degree of the bleeding was assessed by the<br />
number of the pads used and hemoglobin<br />
estimation before and after treatment. 52<br />
patients received monthly SC injection of<br />
Goserelin acetate 3.6 mg (Zoladex,<br />
AstraZeneca, UK ) for three months, the second<br />
group was 50 patients received 5 mg of<br />
norethisterone tablets (Primolut N, Schering,<br />
Germany) orally three times daily during the<br />
attack of bleeding and 5 mg daily if there is no<br />
bleeding to complete 21 days per cycle for<br />
three cycles. Twelve patients failed to complete<br />
the study, two from the goserelin group and ten<br />
from the norethisterone group, so the final<br />
number was 50 in the goserelin group and 40 in<br />
the norethisterone group.The measurement of<br />
the fibroid was done by taking two dimensions<br />
of the largest fibroid, including the biggest<br />
diameter using ultrasound with a convex 3.5<br />
MHz probe (Hunda, Japan) pretreatment and<br />
after three months. Pretreatment hemoglobin<br />
was checked, and then after three months.<br />
General investigations were carried out for both<br />
groups including complete blood picture,<br />
fasting blood sugar, blood urea, and liver<br />
function tests. Patient's acceptance and<br />
response were subjectively registered on a<br />
special questionnaire with any side effects<br />
occurred in that period.The results were<br />
statistically analyzed, using the Statistical<br />
Package for Social Sciences (SPSS) version 11.<br />
Descriptive statistical analyses (frequency<br />
distributions and percentages) were used, while<br />
inferential statistics limited to t test, for<br />
comparison of means, and chi-square test of<br />
proportions. P
Iraqi J Pharm Sci, Vol.19(2) 2010 Goserlin in the management of fibroid<br />
difference reduction in number in the goserelin<br />
group, but not in the norethisterone group.<br />
Table 2 : The effect of goserelin (group 1)<br />
and norethisterone (group 2)<br />
Treatment P value<br />
Groups<br />
Before After<br />
Size o f<br />
fibroid (cm 2 )<br />
Mean<br />
SD<br />
Hb level<br />
(mg/dl)<br />
Mean<br />
SD<br />
Number o f<br />
Pads changed<br />
per day<br />
Group I<br />
Group II<br />
Group I<br />
Group II<br />
Group I<br />
≤5<br />
6-7<br />
8-9<br />
≥10<br />
Group II<br />
≤5<br />
6-7<br />
8-9<br />
≥10<br />
5<br />
8<br />
23<br />
14<br />
4<br />
6<br />
21<br />
9<br />
28.24<br />
6.14<br />
26.56<br />
5.96<br />
9.28<br />
2.44<br />
10.08<br />
2.86<br />
12.3<br />
3.45<br />
25.22<br />
5.01<br />
11.2<br />
1.88<br />
10.24<br />
2.46<br />
29<br />
15<br />
6<br />
0<br />
6<br />
13<br />
18<br />
3<br />
0.0001<br />
0.2589<br />
0.0001<br />
0.7798<br />
0.000<br />
0.102<br />
Table-3 showed the side effects of both groups,<br />
there was no statistical significant difference<br />
between the two groups regarding all the side<br />
effects ( P= 0.119 ), but there was more<br />
menopausal symptoms in the goserelin group,<br />
15 versus 7 in the norethisterone group.Table-4<br />
showed the operations that were done after<br />
finishing the treatment up to about one year.<br />
There were more operations in the<br />
norethisterone group.Twenty five (50%) of<br />
patients in the goserelin group had no<br />
operations versus four (10%) in the<br />
norethisterone group. Seven had hysterectomy<br />
in the goserelin group and 12 in the<br />
norethisterone group and myomectomy in<br />
nineteen and twenty four respectively. All<br />
operations showed statistical significant<br />
reduction in the goserelin group.<br />
Table 3 : the side effects of Gose relin and the<br />
Norethisterone treated groups<br />
Side e ffect Goserelin Nore thisterone P<br />
group group value<br />
No. ( % ) No. ( % )<br />
Menopausa<br />
l symptoms<br />
15 (30 ) 7 ( 17.5 )<br />
Joint pain 13 (26) 5 ( 12.5 )<br />
Skin<br />
allergy<br />
7 (14) 4 ( 10 )<br />
Increase<br />
weight<br />
5 (10 ) 6 ( 15 )<br />
acne 4 ( 8 ) 6 ( 15 )<br />
No<br />
complaint<br />
6 (12 ) 12 ( 30 )<br />
56<br />
total 50 40<br />
Table 4 : the ope rations in both groups<br />
Type of surgery Group 1 Group 2 P value<br />
Hysterectomy 7 12<br />
Myomectomy 18 24 0.000<br />
No operation 25 4<br />
Discussion<br />
The (GnRH) agonists are an effective<br />
medical approach for the management of both<br />
dysfunctional uterine bleeding (DUB) and<br />
uterine fibroids. However, their use is restricted<br />
to short courses due to its long effect on bone<br />
mass. (6) Norethisterone is a common treatment<br />
of menorrhagia in our clinical practice; it is<br />
cheap, always available and well tolerated by<br />
the patients. After the introduction of Goserelin<br />
in our clinical practice, we designed this study<br />
to compare both drugs in cases of menorrhagia<br />
with uterine fibroids. Regarding blood loss, the<br />
increase in haemoglobin is significant after<br />
Goserelin use, (7) (8) with about 1 and 1.5<br />
gm/100ml increase in both studies respectively.<br />
In our study the increase was about 2 gm/100<br />
ml in the Goserelin group, it was not significant<br />
in the norethisterone group. In assessing the<br />
blood loss we use the subjective method by<br />
patient observation in comparison with her<br />
previous menses and the objective methods by<br />
counting pads and hemoglobin estimation, all<br />
showed significant improvement in the<br />
Goserelin group. Ranke HR, found no<br />
significant difference in adding<br />
estradiol/norethisterone to goserelin in<br />
reduction of blood loss. (9) Pre operative<br />
goserelin has been shown to decrease blood<br />
transfusion during operation and increase the<br />
post operative hemoglobin. (10) Goserelin was<br />
found to decrease the size of uterine fibroids.<br />
(11-15) In our study we used two dimensions<br />
measurement of the fibroid by ultrasound, as it<br />
can be done by the usual ultrasound equipment<br />
available in gynecology clinics; the reduction in<br />
the area of the fibroid was from 28.24± 6.14<br />
cm 2 to 12.3±3.45 which represent more than<br />
50%. Bozzini, 2004 found in a randomized<br />
controlled trial that Goserelin use in monthly<br />
injection for 3 months reduce the size of fibroid<br />
by 43%. (16) Adding goserelin after uterine<br />
artery embolization was found not to increase<br />
in the reduction of the size of leiomyoma, (17) in<br />
the same study the reduction of size of fibroid<br />
in Goserelin group was 45%. In a study done<br />
by Lumsden, 1994, on 71 ladies scheduled for<br />
hysterectomy for fibroid, and were divided in 2<br />
groups, one was given Goserelin and the other<br />
placebo before operation and they found that<br />
the size of the fibroid is smaller in the
Iraqi J Pharm Sci, Vol.19(2) 2010 Goserlin in the management of fibroid<br />
Goserelin group, also the haemoglobin level<br />
and the duration of the operation. (18) Goserelin<br />
was also found to increase pregnancy rates if<br />
given before hysteroscopic resection of fibroid<br />
in cases of sub fertility. (19) Many studies found<br />
that blood loss during operation for fibroid after<br />
Goserelin use was less than without it. (17)(20)<br />
Recent study showed that the use of triple<br />
tourniquets is associated with less blood loss<br />
than the use of pre-operative Goserelin in open<br />
myomectomy. (21) Goserelin was found also to<br />
shorten the operative time. (13) In our study<br />
these parameters were not measured.No<br />
significant medical problems were found after<br />
the short time use of Goserelin. (4) In our study<br />
there was no significant difference in the side<br />
effects of both groups (P=0.119), but more<br />
vaso-motor symptoms noted in the Goserelin<br />
group, 39% versus 17.3% in the n<br />
group.Regarding the need for operations, 25 of<br />
the Goserelin group ( 50% ) had operations, 7<br />
hysterectomy and 18 myomectomy, versus 36<br />
out of 40 in the norethsterone group, 12<br />
hysterectomy and 24 myomectomy, so the need<br />
for operation is decreased with Goserelin ,<br />
mostly due to improvement of symptoms, and<br />
the desire of most of the women to preserve the<br />
uterus. Hysterectomy rates for leiomyoma<br />
decreased significantly from 2.13 per 1000 to<br />
1.91 (P < .0001), due to increase in uterine<br />
artery embolization and uterine ablation. (22)<br />
GnRH agonists shrink the uterus and fibroids,<br />
and this effect make it possible to change some<br />
of abdominal hysterectomies to vaginal<br />
hysterectomy, in one study, 76% of GnRH<br />
agonist-treated patients had vaginal<br />
hysterectomy versus 16% of non treated<br />
patients. (23) The use of GnRH agonists can<br />
make possible a conversion from abdominal<br />
hysterectomy to either vaginal hysterectomy or<br />
laparoscopic-assisted vaginal hysterectomy or<br />
laparoscopic supracervical hysterectomy. (23) In<br />
conclusion, Goserelin for three months is a<br />
reasonable choice of treatment for patients<br />
having menorrhagia with uterine fibroid, as it<br />
increases the hemoglobin concentration,<br />
decrease the need for operation, decrease blood<br />
transfusion during operation, and with limited<br />
side effects.<br />
References<br />
1. Buttram, V. and Reiter, R. Uterine<br />
leiomyomata: etiology, symptomatology<br />
and management. Fertil. Steril: 1981; 36,<br />
433–445.)<br />
2. AHRQ (2000) Management of uterine<br />
fibroids. Evidence report. Technology<br />
assessment: Number 34. Agency for<br />
Healthcare Research and Quality of Life.<br />
http://www.ahcpr.gov/<br />
http://www.ahcpr.gov/..<br />
57<br />
3. Crosignani PG, Vercellini P, Meschìa M,<br />
Oldani S, Bramante T GnRH agonists<br />
before surgery for uterine leiomyomas.<br />
Report Med 1996 ; 41(6):415-21.)<br />
4. Cagnacci A, Paoletti AM, Soldani R,<br />
Angiolucci M, Arangino S, Falqui A, Melis<br />
GB Role of goserelin-depot in the clinical<br />
management of uterine fibroids. Obstet<br />
Gynecol 1994; 21(4):263-5.)<br />
5. Irvine GA; Campbell-Brown MB; Lumsden<br />
MA; Heikkila A; Walker JJ; Cameron IT,<br />
Randomised comparative trial of the<br />
levonorgestrel intrauterine system and<br />
norethisterone for treatment of idiopathic<br />
menorrhagia. Br J Obstet Gynaecol<br />
1998;105(6):592-8.<br />
6. Thomas EJ, Add-back therapy for long-term<br />
use in dysfunctional uterine bleeding and<br />
uterine fibroids. Br J Obstet Gynaecol.<br />
1996; 103 Suppl 14:18-21<br />
7. Gerris J, Degueldre M, Peters AA, Romao<br />
F, Stjernquist M, al-Taher H<br />
The place of Zoladex in deferred surgery for<br />
uterine fibroids. Zoladex Myoma Study<br />
Group.] Horm Res 1996; 45(6):279-84.<br />
8. Stovall TG, Summit RL, Washburn SA,<br />
Ling FW Gonadotropin-releasing hormone<br />
agonist use before hysterectomy. Am J<br />
Obstet Gynecol 1994 ; 170(6):1744-8;<br />
discussion 1748-51.<br />
9. Franke HR, Snaaijer FF, Houben PW, van<br />
der Mooren MJ Treatment of dysfunctional<br />
uterine bleeding in the perimenopause: The<br />
effects of adding combined estradiol /<br />
norethisterone acetate therapy to goserelin<br />
acetate treatment: Gynecol Endocrinol<br />
2006 ; 22(12):692-7<br />
10. Lim SS, Sockalingam JK, Tan PC:<br />
Goserelin versus leuprolide before<br />
hysterectomy for uterine fibroids. Int J<br />
Gynaecol Obstet 2007 27.<br />
11. Moris,EP, RymerJ, RobinsonJ, FogelmanI<br />
Efficacy of tibolone as "add-back therapy"<br />
in conjunction with a gonadotropinreleasing<br />
hormone analogue in the treatment<br />
of uterine fibroids. Fertil Steril 2007 15.<br />
12. Russo P, Ciolli P, Atlante M, Carico E,<br />
Mancini R, Russo R, Vecchione A [Clinical<br />
use of leuprolide acetate depot in a group of<br />
gynecologic patients in the preoperative<br />
period; Minerva Ginecol 1998 ; 50(11):499-<br />
502.<br />
13. Tiufekchieva E, Nikolov A:<br />
Hysteroresection of submucous myomas<br />
after treatment with zoladex; Akush<br />
Ginekol (Sofiia) 2006; 45(1):19-24.<br />
14. Donnez J, Hervais Vivancos B, Kudela M,<br />
Audebert A, Jadoul P A randomized,<br />
placebo-controlled, dose-ranging trial<br />
comparing fulvestrant with goserelin in
Iraqi J Pharm Sci, Vol.19(2) 2010 Goserlin in the management of fibroid<br />
premenopausal patients with uterine fibroids<br />
awaiting hysterectomy. Fertil Steril 2003 ;<br />
79(6):1380-9.<br />
15. Baytur YB, Ozbilgin K, Cilaker S, Lacin S,<br />
Kurtul O, Oruc S, Koyuncu FM A<br />
comparative study of the effect of raloxifene<br />
and gosereline on uterine leiomyoma<br />
volume changes and estrogen receptor,<br />
progesterone receptor, bcl-2 and p53<br />
expression immunohistochemically in<br />
premenopausal women. Eur J Obstet<br />
Gynecol Reprod Biol 2006 Sep 12<br />
16. BozziniN, MessinaML, BorsariR,<br />
HilarioSG, PinottiJA Comparative study of<br />
different dosages of goserelin in size<br />
reduction of myomatous uteri.J Am Assoc<br />
Gynecol Laparosc 2004 ;11(4): 462-3.I<br />
17. VilosGA,VilosAG, , Abu-RafeaB, PronG,<br />
KozakR, GarvinG, Administration of<br />
goserelin acetate after uterine artery<br />
embolization does not change the reduction<br />
rate and volume of uterine myomas. Fertil<br />
Steril 2006 ; 85(5):1478-83.<br />
18. Lumsden MA, West CP, Thomas E, Coutts<br />
J, Hillier H, Thomas N, Baird DT<br />
Treatment with the gonadotrophin releasing<br />
58<br />
hormone-agonist goserelin before<br />
hysterectomy for uterine fibroids. Br J<br />
Obstet Gynaecol 1994 ; 101(5):438-42.<br />
19. Narayan R, Rajat, Goswamy K Treatment<br />
of submucous fibroids, and outcome of<br />
assisted conception. J Am Assoc Gynecol<br />
Laparosc 1994 ;1(4 Pt 1):307-11.<br />
20. Crosignani PG, Vercellini P, Meschìa M,<br />
Oldani S, Bramante T GnRH agonists<br />
before surgery for uterine leiomyomas. ]J<br />
Reprod Med 1996 ; 41(6):415-21<br />
21. Al-Shabibi N, Chapman L, Madari S,<br />
Papadimitriou A, Papalampros P, Magos A<br />
Prospective randomised trial comparing<br />
gonadotrophin-releasing hormone analogues<br />
with triple tourniquets at open<br />
myomectomy. BJOG 2009 Feb 4.<br />
22. Jacobson GF, Shaber RE, Armstrong MA,<br />
Hung YY. Links Changes in rates of<br />
hysterectomy and uterine conserving<br />
procedures for treatment of uterine<br />
leiomyoma. . Am J Obstet Gynecol. 2007;<br />
196(6):601.e1-5; discussion 601.e5-6.<br />
23. Carter JE. Alternatives to total abdominal<br />
hysterectomy. JSLS. 1997; 1:259-262.
Iraqi J Pharm Sci, Vol.19(2) 2010<br />
Anti-bacterial Properties of Melatonin against Mycobacterium<br />
Tuberculosis in Vitro<br />
Thamer M. Jasim*, Mustafa G.Alabbassi** , Suhad F. Hatem Almuqdadi*<br />
and Jinan K. Kamel***<br />
* Department of Microbiology and Biotechnology,Al-Mustansyria,University,College of Pharmacy,<br />
Baghdad, Iraq.<br />
** Department of Pharmacology and Therapeutics, Al-Mustansyria University,College of Pharmacy,<br />
Baghdad, Iraq.<br />
*** National Reference Laboratory<br />
Abstract<br />
57 isolates of Mycobacterium tuberculosis and Mycobacterium bovis were identified; they were<br />
isolated from different clinical sources which included sputum, bronchial wash, abscess, pleural fluid,<br />
gastric fluid, eye fluid, and CSF, also urine and ear swab. This investigation was carried out on 198<br />
patient attended National Reference Laboratory for T.B during September 2009. Also the study<br />
declared that the ratio of separation of this bacterium from male was (67.6%) and it’s higher than the<br />
ratio of separation this bacterium from females which was (32.3%). The susceptibility of<br />
Mycobacterium tuberculosis to melatonin was evaluated. Many concentrations of melatonin were<br />
prepared to investigate it as antibacterial drug against multidrug resistant Mycobacterium tuberculosis<br />
and Mycobacterium bovis. Suspension bacteria (10 -1 , 10 -3 and 10 -5 ) were cultured on Lowenstein-<br />
Jensen media (LJ) contains melatonin, while control media without this drug. Six isolates were chosen<br />
according to their susceptibility patterns; they were resisting to Rifampicin, Streptomycin, Isonicotinic<br />
–hydrazide and sensitive to Ethambutol. In conclusion, these in vitro studies clearly demonstrate antibacterial<br />
effects of melatonin. Among possible mechanisms, it is concluded that melatonin showed<br />
antibacterial effects against multidrug resistant T.B by reducing intracellular substrates. Identifying the<br />
mode of action could be of great help in developing and researching new anti-bacterial drugs.<br />
Key words: Antibacterial, Melatonin, Mycobacterium tuberculosis.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﻲﺘﻟﺍﻭ ﺔﻔﻠﺘﺨﻤﻟﺍ ﺔﻳﺮﻳﺮﺴﻟﺍ ﺭﺩﺎﺼﻤﻟﺍ ﻦﻣ ﺔﻳﺮﻘﺒﻟﺍ ﺔﻴﻧﺭﺪﻟﺍ ﺕﺎﻴﺼﻌﻟﺍﻭ ﺔﻳﺮﻄﻔﻟﺍ ﺔﻴﻧﺭﺪﻟﺍ ﺕﺎﻴﺼﻌﻟﺍ ﻦﻣ ﺔﺒﺟﻮﻣ ﺔﻟﺰﻋ 57 ﺺﻴﺨﺸ ﺗ ﻢﺗ<br />
ﻰﻠﻋ ﺔﺳﺍﺭﺪﻟﺍ ﻩﺬﻫ ﺕﺬﻔﻧ . ﻥﺫﻷﺍ ﺔﺤﺴﻣﻭ ﺭﺍﺭﺩﻻﺍ ﻚﻠﻟﺬﻛ ﻲﻋﺎﺨﻨﻟﺍﻭ ﻦﻴﻌﻟﺍﻭ ﺓﺪﻌﻤﻟﺍﻭ ﺐﻨﺠﻟﺍ ﻞﺋﺎﺳ ٬ﺝﺍﺮﺨﻟﺍ ٬ﻲﺒﺼﻘﻟﺍ ﻞﺴﻐﻟﺍ ٬ ﻊﺸﻘﻟﺍ ﺖﻨﻤﻀﺗ<br />
ﻲﻫ (% 67.6)<br />
ﺭﻮﻛﺬﻟﺍ<br />
ﺔﺑﺎﺻﺍ ﺔﺒﺴﻧ ﻥﺍ ﺔﺳﺍﺭﺪﻟﺍ ﺖﺤﺿﻭﺃ<br />
. 2009 ﻝﻮﻠﻳﺍ ﺮﻬﺷ ﻝﻼﺧ ﻥﺭﺪﺘﻠﻟ<br />
ﻲﻌﺟﺮﻤﻟﺍ ﻲﻨﻃﻮﻟﺍ ﺮﺒﺘﺨﻤﻟﺍ<br />
ﻊﺟﺍﺭ ﺾﻳﺮﻣ 198<br />
ﻦﻴﻧﻮﺗﻼﻴﻤﻟﺍ ﻦﻣ ﺰﻴﻛﺍﺮﺗ ﺓﺪﻋ ﻡﺍﺪﺨﺘﺳﺍ ﻢﺗ . ﻦﻴﻧﻮﺗﻼﻴﻤﻟﺍ ﺭﺎﻘﻌﻟ ﺔﻴﻧﺭﺪﺘﻟﺍ ﺕﺎﻴﺼﻌﻟﺍ ﺔﻴﺳﺎﺴﺣ ﻢﻴﻴﻘﺗ ﻢﺗ (% 32.3)<br />
ﺙﺎﻧﻷﺍ ﺔﺑﺎﺻﺍ ﺔﺒﺴﻧ ﻦﻣ ﻰﻠﻋﺍ<br />
ﻒﻴﻓﺎﺨﺗ ﺓﺪﻋ ﺖﻋﺭﺯ<br />
. ﺕﺍﺩﺎﻀﻤﻠﻟ ﺓﺩﺪﻌﺘﻤﻟﺍ ﺔﻣﻭﺎﻘﻤﻟﺍ ﺕﺍﺫ M. bovis ﻭ M. Tuberculosis ﻥﺭﺪﺘﻟﺍ ﺕﺎﻴﺼﻋ ﺪﺿ ﺎﻳﺮﺘﻜﺒﻠ ﻟ ﺩﺎﻀﻤﻛ<br />
( 10 ﻱﺮﻴﺘﻜﺒﻟﺍ ﻖﻟﺎﻌﻠﻟ<br />
-5 ,10 -3 ,10 -1 ﺍﺬﻫ ﻱﻮﺤﻳﻻ ﺓﺮﻄﻴﺴﻟﺍ ﻂﺳﻭ ﺎﻤﻨﻴﺑ ﻦﻴﻧﻮﺗﻼﻴﻤ ﻟﺍ ﺭﺎﻘﻋ ﻰﻠﻋ ﻱﻮﺘﺤﻳ ﻱﺬﻟﺍﻭ (LJ) ﻂﺳﻭ ﻰﻠﻋ<br />
)<br />
٬ﻦﻴﺴﻳﺎﻣﻮﺘﺑﺮﺘﺴﻟﺍ ٬ﻦﻴﺴﺒﻣﺎﻔﻳﺮﻠﻟ ﺔﻣﻭﺎﻘﻣ ﺕﻻﺰﻌﻟﺍ ﻩﺬﻫ ﺖﻧﺎﻛ ﺚﻴﺣ ﺔﻴﺴﺴﺤﺘﻟﺍ ﻁﺎﻤﻧﻷﺍ ﻰﻠﻋ ﺩﺎﻤﺘﻋﻷﺎﺑ ﺕﻻﺰﻋ ﺔﺘﺳ ﺕﺮﻴﺘﺧﺍ . ﺭﺎﻘﻌﻟﺍ<br />
ﺎﻳﺮﺘﻜﺒﻟﺍ ﻰﻠﻋ ﻦﻴﻧﻮﺗﻼﻴﻤﻠﻟ ﻲﻄﻴﺒﺜﺘﻟﺍﺮﻴﺛﺎﺘﻟﺍ<br />
ﺔﺳﺍﺭﺪﻟﺍ ﻩﺬﻫ ﻦﻣ ﺞﺘﻨﺘﺴﻧ . ﻝﻮﺘﻴﺒﻣﺎﺜﻳﻼﻟ ﺔﺳﺎﺴﺣﻭ ٬INHﺪﻳﺍﺯﺍﺭﺪﻳﺎﻫ<br />
ﻚﻨﺗﻮﻜﻴﻧﻭﺰﻳﺍ<br />
. ﺪﻳﺪﺟ ﻱﺮﻴﺘﻜﺑ ﺩﺎﻀﻤﻟ ﻕﺎﻓﻵﺍ<br />
ﺢﺘﻔﻳ ﻚﻠﻟﺬﺑﻭ ﺎﻳﺮﺘﻜﺒﻠﻟ ﺔﻴﻠﺨﻟﺍ ﻞﺧﺍﺩ ﻰﻠﻋ ﻩﺮﻴﺛﺄﺗ<br />
ﺔﻄﺳﺍﻮﺑ ﺔﻠﻤﺘﺤﻤﻟﺍ ﺔﻴﻜﻴﻧﺎﻜﻴﻤﻟﺍﻭ ﺎﻳﺮﺒﺘﺨﻣ<br />
Introduction<br />
Although tuberculosis (TB) is a<br />
preventable and treatable disease, it causes 3<br />
million deaths annually. The current situation<br />
of TB is unique, mainly due to two aspects: the<br />
association between TB and human<br />
immunodeficiency virus (HIV) infection, and<br />
the global spread of virulent strains resistant to<br />
key antituberculous drugs. There is a direct<br />
association between HIV infection and the<br />
reactivation of latent TB or the progression of<br />
TB following newly acquired infection (1) .<br />
Melatonin originally identified as an effector<br />
for circadian rhythms, is now known to be a<br />
hormone involved in a vast range of<br />
homeostasis maintenance activities, for<br />
example seasonal timing, sexual development,<br />
the antioxidant defense system and immune<br />
1Corresponding author E- mail :<br />
Received : 10/4/2010<br />
Accepted : 18/9/2010<br />
59<br />
response (2) . Melatonin is synthesized from<br />
tryptophan within the serotonin pathway<br />
mainly in the pineal gland, and in a number of<br />
extrapineal organs such as retina, lens, bone<br />
marrow, intestine, skin and so on. To date,<br />
three mammalian melatonin receptors, Gprotein<br />
coupled receptors MT1 and MT2, and a<br />
quinone reductase family receptor, MT3, have<br />
been identified (3) . Melatonin is a highly<br />
studied endogenous molecule. This indolamine<br />
has a variety of beneficial effects within cells<br />
and organisms, including cell cycle regulation<br />
(4) . Although there are a plethora of studies on<br />
melatonin, only a few of them relate to it in<br />
vitro antimicrobial activities (5–7) on to its<br />
effects in infectious diseases (8-10) .
Iraqi J Pharm Sci, Vol.19(2) 2010<br />
New in vitro antimicrobial studies using<br />
melatonin suggested that it has limited<br />
antimicrobial properties (11, 12) while one study<br />
found that melatonin inhibited Candida<br />
albicans at a concentration of 300 µg/ml (12) .In<br />
contrast to the in vitro studies, virtually all in<br />
vivo studies performed with melatonin in<br />
infectious disease models documented it as a<br />
successful therapy (9, 13) . Melatonin is a highly<br />
versatile molecule. One example is its ability<br />
to limit the growth of a variety of tumor types.<br />
One of several proposed mechanisms to<br />
explain melatonin’s inhibitory actions on<br />
cancer growth is its ability to curtail the uptake<br />
of growth factors which promote cell<br />
proliferation<br />
(14) . Linoleic acid (LA), an<br />
essential omega-6 polyunsaturated fatty acid is<br />
a growth factor for a number of tumor types.<br />
Via an action on the cell membrane, melatonin<br />
prevents the uptake of this fatty acid by cancer<br />
cells which reduces the activation of genes that<br />
promote cell proliferation (15) . Similar actions<br />
of melatonin on the bacterial wall thus may<br />
restrict the survivability of bacteria.<br />
Additionally, melatonin has a high metal<br />
binding capacity. Melatonin binds iron (III),<br />
copper and zinc thereby reducing their<br />
cytoplasmic availability. Bacteria are strongly<br />
dependent on free metals, in particular, free<br />
iron for growth (16) . Clearly, there are several<br />
potential mechanisms that may explain the<br />
possible antibacterial efficacy of melatonin. In<br />
the current study we tested the antimicrobial<br />
effects of melatonin against resistant strains of<br />
Mycobacterium tuberculosis.<br />
Materials and Methods<br />
Samples<br />
A total of 198 clinical samples from<br />
National Reference Laboratory for T.B, were<br />
included in this study. They were collected<br />
during November 2009. Identification of these<br />
isolates as Mycobacterium tuberculosis and<br />
Mycobacterium bovis were based on<br />
biochemical properties (17) . The most samples<br />
were sputum; they are transfer to the<br />
laboratory in sterile container. Three samples<br />
from each patient were taken to diagnose<br />
tuberculosis. Susceptibility test was done to 13<br />
isolates from 57 positive samples to<br />
tuberculosis according to routine work of<br />
national reference laboratory for tuberculosis.<br />
All samples treat with sodium hydroxide and<br />
hydrochloric acid to remove all<br />
microorganisms and epithelial cells (Petroffs<br />
method). Zeihl-Neelsen stain was done for all<br />
specimens.<br />
Culture the Specimens<br />
The specimens was cultured on<br />
Lowenstein-Jensen media (LJ) pouring in<br />
60<br />
screw –capped tubes in final volume 6ml, these<br />
tubes put in oven at 80-85 C for 45 min. The<br />
media left for 24 hr to be insure that there is no<br />
contamination. The treated specimens will<br />
culturing by using Pasture pipette, (0.4-0.2) ml<br />
from inoculme of specimen must be transferred<br />
to LJ media and let the culture for 72 hr at 37<br />
C in incubators( in slope position), then for 50<br />
days at 37 C ( in vertical position).<br />
Susceptibility Test<br />
Susceptibility test was done by using the<br />
proportional method (18) , four antibiotic<br />
solutions were used; Rifampicin 40 µg/ml,<br />
Streptomycin 4 µg/ml, Ethambutol 2 µg/ml,<br />
Isonicotinic hydrazide 0.2 µg/ml.<br />
Mycobacterium tuberculosis suspension was<br />
prepared in many dilutions 10 -1 -10 -5, the<br />
primary dilution adjusted with MacFerland<br />
solution(3×10 8 ) CFU/ml. 100 µl from 10 -1 , 10 -<br />
3 and 10 -5 dilution was cultured on media (LJ)<br />
contain antibiotics and without antibiotics as<br />
control. After 6-8 weeks the result must be<br />
read, if the growth on media contains<br />
antibiotics more than control media this means<br />
resistant bacteria. While the growth consider<br />
sensitive when the growth less than control<br />
media, for this test we chose 4 isolates from<br />
Mycobacterium tuberculosis and 2 isolates<br />
from Mycobacterium bovis, according to their<br />
typing (catalase, nitrase test, niacin test and<br />
colony form).<br />
Melatonin drug<br />
In this study, many concentration of<br />
melatonin dissolved in 96% ethanol (0.4, 0.2,<br />
0.1, 0.05) mg/ml were prepared to investigate<br />
it as antibacterial drug against multi-drug<br />
resistant Mycobacterium tuberculosis and<br />
Mycobacterium bovis.Suspension bacteria (10 -<br />
1 , 10 -3 and 10 -5 ) were cultured on LJ media<br />
contains melatonin, while control media<br />
without this drug. Six isolates were chosen<br />
according to their susceptibility patterns, they<br />
were resist to Rifampicin, Streptomycin,<br />
Isonicotinic –hydrazide and sensitive to<br />
Ethambutol (19) .<br />
Results<br />
Table -1 showed us the number of<br />
Mycobacterium tuberculosis isolated from<br />
many sources. It was found that 57 samples<br />
were positive samples. Table-2 shows number<br />
and percentage of infection with M.<br />
tuberculosis and M. bovis according to the sex,<br />
it was found that 134(67.6%) of patients were<br />
males and 64(32.3%) were females with<br />
present of statistical significant. Table-3<br />
shows the percentage of infected with M.<br />
tuberculosis and M.bovis according to the age.<br />
The higher percentage rate (46.2%) was found<br />
in the group of (30-39 year) while the lowest
Iraqi J Pharm Sci, Vol.19(2) 2010<br />
percentage rate (6.7%) was found in the group<br />
(70≥ year) with present of statistical<br />
differences. Table-3 shows the number and<br />
percentage of infection with Mycobacterium<br />
SPP. According to the sex, it was found that<br />
134 (67.6%) of patients were males and<br />
64(32.3%) were females with present of<br />
statistical significant differences. Table-4<br />
shows the effect of many concentration of<br />
melatonin on M. tuberculosis and M.bovis<br />
isolates. It was found that M. tuberculosis and<br />
M.bovis sensitized to the concentrations 0.05,<br />
0.1, 0.2 and 0.4mg/ml. Table-5 shows the<br />
susceptibility patterns of many dilution of M.<br />
tuberculosis and M. bovis to different<br />
concentration of melatonin. It was found that<br />
dilution of bacteria 10 -1 , 10 -3 and 10 -5 were<br />
sensitized to all concentrations of melatonin<br />
that were used. Figure -1 shows the growth of<br />
M. tuberculosis and M bovis. Resistant to<br />
Rifampicin, streptomycin and INH and were<br />
sensitize to Ethambutol.and Melatonin.<br />
Table 1 : Number of M.tuberculosis and<br />
M.bovis isolated from many sources<br />
Isolates source<br />
Numbe r of<br />
Examine d<br />
Samples<br />
Numbe r<br />
Of Positive<br />
Sample<br />
No. (%)<br />
Sputum 145 55 96.5<br />
Bronchial wash 19 1 1.8<br />
Abscess 5 1 1.8<br />
Pleural Fluid 13 - -<br />
Gastric Fluid 3 - -<br />
Eye Fluid 2 - -<br />
Knee Fluid 1 - -<br />
C.S.F 1 - -<br />
Ear Swabs 1 - -<br />
Urine 8 - -<br />
Total 198 57<br />
Table 2 : Number and perce ntage of<br />
infe ction with M. tuberculosis and M. bovis<br />
according to the sex<br />
Sex<br />
of<br />
Patients<br />
Examined<br />
samples<br />
Number<br />
of positive<br />
M.<br />
tuberculosis<br />
iso lates<br />
Number<br />
of positive<br />
M. bovis<br />
isolates<br />
No. % No. % No. %<br />
Male 134 67.7 23 65.7 14 63.6<br />
female 64 32.3 12 34.3 8 36.4<br />
Total 198 35 22<br />
61<br />
Table 3 : Percentage of infected with M.<br />
tuberculosis and M.bovis according to age .<br />
Age<br />
Groups<br />
Numbe r of<br />
Examined<br />
Samples<br />
Number of Positive<br />
Sample<br />
No. (%)<br />
10< year 6 0 0<br />
10-19 year 16 6 *37.5 %<br />
20-29 year 36 14 38.9 %<br />
30-39 year 39 18 46.2 %<br />
40-49 year 29 6 37.5 %<br />
50-59 year 41 8 19.5 %<br />
60-69 year 16 4 25 %<br />
70≥ year 15 1 **6.7 %<br />
Total 198 57<br />
** Statistically significant difference<br />
Table 4 : Effect of many conce ntration of<br />
melatonin on Mycobacterium SPP. Isolates<br />
Code Me latonin concentration (mg/ml)<br />
of<br />
isolates<br />
(0.4) (0.2) (0.1) (0.05) Control<br />
S S S S R<br />
L2 S S S S R<br />
L3 S S S S R<br />
L4 S S S S R<br />
L5 *<br />
S S S S R<br />
L6 *<br />
S S S S R<br />
L =isolate, S =Sensitive, R=Resist, *= M. bovis<br />
Table 5 : Susceptibility patterns of many<br />
dilutions M.tuberculosis and M.bovis to<br />
many concentration of Melatonin<br />
Dilution<br />
of<br />
Bacteria<br />
3×10 7<br />
CFU/ml<br />
(10 -1 )<br />
3×10 5<br />
CFU/ml<br />
(10 -3 )<br />
3×10 3<br />
CFU/ml<br />
(10 -5 )<br />
Melatonin concentration<br />
mg/ml<br />
(0.4) (0.2) (0.1) (0.05) Contro l<br />
S S S S R<br />
S S S S R<br />
S S S S R
Iraqi J Pharm Sci, Vol.19(2) 2010<br />
Figure 1 : Growth of M.tube rculosis resists<br />
to Rifampicin,Stre ptomycin, and INH and<br />
was sensitized to Ethambutol and Me latonin<br />
on a Lowesten agar slant (from the left to<br />
the right).<br />
Discussion<br />
The results of the present study indicate<br />
that melatonin has in vitro antimicrobial<br />
activity against strains of antibiotic-resistant<br />
mycobacterium tuberculosis. As melatonin is<br />
weakly soluble in water, investigators<br />
generally use ethanol to dissolve the<br />
indolamine. The antibacterial effects of<br />
melatonin could be a result of the metal<br />
binding capacity of the indolamine. Normally,<br />
tissue fluids contain unsaturated iron-binding<br />
proteins including transferrin in plasma and<br />
lymph and lactoferrin in other secretions such<br />
as milk or mucous (20, 21) . These proteins ensure<br />
that the concentration of free iron in these<br />
fluids is virtually zero. This is essential for the<br />
normal bactericidal and bacteriostatic effects<br />
of plasma and extracellular fluids. If iron<br />
becomes freely available, the antibacterial<br />
effects of these fluids are lost. This can lead to<br />
rapid extracellular bacterial growth and a<br />
major increase in bacterial virulance.<br />
Pathogenic bacteria have also ways of<br />
extracting essential iron from the low iron<br />
environment in vivo via siderophores such as<br />
enterochelin, which can remove iron from<br />
unsaturated transferrin or lactoferrin (22) . An<br />
intriguing aspect of this issue is that, because<br />
iron is absolutely essential for bacteria, it could<br />
make the development of bacterial resistance<br />
very difficult for any organism deprived of<br />
iron. Of the concern being expressed over the<br />
increasing resistance to antibiotics now being<br />
encountered, particularly within hospitals,<br />
studies on the development of novel drugs<br />
against both iron transport and/or intrabacterial<br />
free iron availability should be undertaken.<br />
Melatonin reportedly has a high metal binding<br />
capacity including iron. Limson et al. (23)<br />
62<br />
observed that melatonin and its precursors<br />
exhibited the ability to bind metals in situ.<br />
Gulcin et al. (24) also noted that melatonin is an<br />
effective metal chelating agent. This feature of<br />
melatonin has been typically thought to be<br />
related to the antioxitant properties of the<br />
indole by making transition metals unavailable<br />
for the Fenton reaction. However, in case of<br />
bacterial growth, an agent which binds free<br />
iron in the cytoplasm has great importance. As<br />
melatonin easily passes all biological barriers<br />
including bacterial cell wall, it may bind free<br />
iron in the cytoplasm and restrict bacterial<br />
growth via this mechanism. In the present<br />
study, melatonin was tested against resistant<br />
mycobacterium tuberculosis. In gram-negative<br />
bacteria, the cell envelope is composed largely<br />
of protein glycopeptide, lipopolysaccharide,<br />
and also, substantial amounts of lipid (25) .<br />
Melatonin has been shown to limit the uptake<br />
of LA and total fatty acids by human breast<br />
cancer cells. This feature may also work<br />
against an extremely fast dividing prokaryote.<br />
Konar et al. (7) reported that melatonin, at the<br />
concentration of 1000 µg/ml, significantly<br />
reduced the lipid level of Sacchoramyces<br />
cerevisae. Furthermore in the same study,<br />
melatonin, at concentration of 300 µg /ml, was<br />
shown to be one of the most effective agents in<br />
reducing lipid levels of Candida albicans. In<br />
organisms, melatonin can be administered via<br />
any of several routes, e.g. orally, sublingually,<br />
etc., and it is available either as an over-thecounter<br />
supplement or as a prescription drug<br />
(depending on the country). The molecule has<br />
a long shelf-life and is inexpensive relative to<br />
conventional drugs used to treat the variety of<br />
bacterial infections.Melatonin is also very safe<br />
with few side effects and a very wide safety<br />
margin. The current in vitro studies should be<br />
expanded to investigate the efficacy of<br />
melatonin as an in vivo antibiotic. It has been<br />
previously shown that melatonin is beneficial<br />
in newborn humans suffering fro m septicemia<br />
(26) . Those findings coupled with the data<br />
reported here suggest further investigations of<br />
the role of melatonin in reducing bacterial<br />
growth.<br />
References<br />
1. Abate G, Hoffner S, Stegard V, Mqrner H.<br />
Characterization of Isoniazide-Resistant<br />
Starins of Mycobacterium tuberculosis on<br />
the basis of Phenotypic Properties and<br />
Mutations in KatG. Eur J Clin Microbiol<br />
Infect Dis , 2001; 20: 239-333.<br />
2. Mohd. A, Yoshinao A, Hajime F, Tomoyuki<br />
M et al. Serotonin and melatonin,<br />
neurohormones for homeostasis, as novel
Iraqi J Pharm Sci, Vol.19(2) 2010<br />
inhibitors of infections by the intracellular<br />
parasite Chlamydia. Journal of<br />
Antimicrobial Chemotherapy,2005;10:1-8.<br />
3. Chan AS, Lai FP, Lo RK et al. Melatonin<br />
MT1 and MT2 receptors stimulate c-Jun<br />
N-terminal kinase via pertussis toxinsensitive<br />
and insensitive G proteins. Cell<br />
Signal, 2002; 14: 249–57.<br />
4. Reiter RJ, Gultekin F, Flores LJ et al.<br />
Melatonin: potential utility for improving<br />
public health. Kor Hek, 2006; 5:131–158.<br />
5. Wang HX, Liu F, NG TB. Examination of<br />
pineal indoles and 6-methoxy-2-<br />
benzoxazolinone for antioxidant and<br />
antimicrobial effects. Comp Biochem<br />
Physiol C Toxicol Pharmacol, 2001;<br />
130:379–388.<br />
6. Ozturk AI, Yilmaz O, Kirbag S, Arslan M.<br />
Antimicrobial and biological effects of<br />
ipemphos and amphos on bacterial and<br />
yeast strains. Cell Biochem Funct, 2000 ;<br />
18 : 117–126.<br />
7. Robertson GT, Doyle TB, Du Q, Duncan<br />
L, Mdluli KE, Lynch AS: A novel indole<br />
compound that inhibits Pseudomonas<br />
aeruginosa growth by targeting MreB is a<br />
substrate for MexAB-OprM. J Bacteriol,<br />
2007; 189: 6870–6881.<br />
8. Grandgirard D, Leib SL. Strategies to<br />
prevent neuronal damage in paediatric<br />
bacterial meningitis. Curr Opin Pediatr,<br />
2006; 18:112–118.<br />
9. Valero N, Espina LM, Mosquera J.<br />
Melatonin decreases nitric oxide<br />
production , inducible nitric oxide<br />
synthase expression and lipid peroxidation<br />
induced by Venezuelan encephalitis<br />
equine virus in neuroblastoma cell<br />
cultures.Neurochem Res , 2006 ; 31: 925–<br />
932.<br />
10. Valero N, Marinaespina L, Bonilla E,<br />
Mosquera J. Melatonin decreases nitric<br />
oxide production and lipid peroxidation<br />
and increases interleukin-1 beta in the<br />
brain of mice infected by the Venezuelan<br />
equine encephalomyelitis virus. J Pineal<br />
Res, 2007; 42:107–112.<br />
11. Wang HX, Liu F, NG TB. Examination of<br />
pineal indoles and 6-methoxy-2-<br />
benzoxazolinone for antioxidant and<br />
antimicrobial effects. Comp Biochem<br />
Physiol C Toxicol Pharmacol, 2001;<br />
130:379–388.<br />
12. Konar VV, Yilmaz O, Ozturk AI et al.<br />
Antimicrobial and biological effects of<br />
bomphos and phomphos on bacterial and<br />
yeast cells. Bio-Organic Chemistry, 2000;<br />
28:214–225.<br />
13. Reynolds FD, Dauchy R, Blask D et al.<br />
The pineal gland hormone melatonin<br />
63<br />
improves survival in a rat model of sepsis/<br />
shock induced by zymosan A. Surgery<br />
2003; 134:474–479.<br />
14. Reiter RJ. Mechanisms of cancer<br />
inhibition by melatonin. J Pineal Res,<br />
2004; 37:213–214.<br />
15. Blask DE, Dauchy RT, Sauer LA. Putting<br />
cancer to sleep at night: the<br />
neuroendocrine / circadian melatonin<br />
signal. Endocrine, 2005; 27:179–188.<br />
16. Ward CG, Bullen JJ, Rogers HJ. Iron and<br />
infection: new developments and their<br />
implications. J Trauma, 1996; 41:356–<br />
364.<br />
17. Vestal AL. In: Procedure for the isolation<br />
and identification of mycobacteria. US<br />
Department of Health, Education and<br />
Welfare Pub no. (CDC) 77 - 8230.<br />
Atlanta, Georgia: Centers for Disease<br />
Control and Prevention; 1977. p. 15-90.<br />
18. Guidelines for surveillance of drug<br />
resistance in TB 1997, WHO, IUATLD.<br />
19. Canetti G, Fox W, Khomenko A, Mahler<br />
HT, Menon NK,Mitchison DA, et al.<br />
Advances in techniques of testing<br />
mycobacterial drug sensitivity and the use<br />
of sensitivity tests in tuberculosis control<br />
programmes. Bull World Health Organ,<br />
1969; 41 : 21-43.<br />
20. Omer Faruk, RecaiOgur1, Ahmet<br />
Korkmaz, Abdullah Kilic,Russel J.Reiter .<br />
Melatonin as an antibiotic: new insights<br />
into the actions of this ubiquitous<br />
molecule. J.Pineal Res, 2008; 44:222–226.<br />
21. Bullen JJ, Rogers HJ, Spalding PB, Ward<br />
CG. Iron and infection: the heart of the<br />
matter. FEMS Immunol Med Microbiol,<br />
2005; 43:325–330.<br />
22. Bullen JJ, Rogers HJ, Spalding PB, Ward<br />
CG. Natural resistance, iron and infection:<br />
a challenge for clinical medicine. J Med<br />
Microbiol 2006; 55:251–258.<br />
23. Limson J, Nyokong T, Daya S. The<br />
interaction of melatonin and its precursors<br />
with aluminium, cadmium, copper, iron,<br />
lead, and zinc: an adsorptive voltammetric<br />
study. J Pineal Res, 1998; 24:15–21.<br />
24. Gulcin I,Buyukokuroglu ME, Kufrevioglu<br />
OI.Metal chelating and hydrogen peroxide<br />
scavenging effects of melatonin. J Pineal<br />
Res 2003; 34:278–281.<br />
25. Hebeler BH, Chatterjee AN, Young FE.<br />
Regulation of the bacterial cell wall: effect<br />
of antibiotics on lipid biosynthesis.<br />
Antimicrob Agents Chemother 1973;<br />
4:346–353.<br />
26. Gitto E, Karbownik M, Reiter RJ et al.<br />
Effects of melatonin treatment in septic<br />
newborns. Pediatr Res, 2001; 50:756–760.
Iraqi J Pharm Sci, Vol.19(2) 2010 Tinidazole bioadhesive vaginal gels<br />
In vitro Evaluation of Tinidazole Bioadhesive Vaginal Gels<br />
, 1<br />
Zainab T.Salih*<br />
*Department of Pharmaceutics, College of Pharmacy, University of Baghdad,Baghdad, Iraq.<br />
Abstract<br />
Many trials were made to prepare Tinidazole 2% as bioadhesive vaginal gels using different gel<br />
bases including hydroxypropyl methyl cellulose (3 and 4% w/w), methylcellulose (3 and 4%w/w) and<br />
carboxymethylcellulose (2 and 3% w/w) .Swelling index of the polymers,pH , viscosity , bioadhesive<br />
force , and in-vitro drug release to the simulating vaginal fluid (S.V.F.) were investigated for all the<br />
prepared bioadhesive gels . The mechanism of drug release from the gel bases was also investigated.<br />
The results revealed that C MC 3% gave the highest viscosity and bioadhesive strength with the lowest<br />
release rate while lowest viscosity and bioadhesive force was obtained with HPMC gel base with the<br />
highest release rate. The mechanism of drug release was affected by the type of gel base. Fickian<br />
diffusion was obtained with all gel bases.<br />
Key words: Tinidaz ole, bioadhesive polymers, gels<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﻞﻴﺜﻣ ﻞﻴﺑﻭﺮﺑ ﻲﺴﻛﻭﺭﺪﻴﻫ ﻞﻤﺸﺗ ﺔﻔﻠﺘﺨﻣ ﺔﻴﻣﻼﻫ ﺪﻋﺍﻮﻗ ﻡﺍﺪﺨﺘﺳﺎﺑ ﻕﺎﺼﺘﻟﻻﺍ ﻲﻃﺎﺨﻣ ﻲﻠﺒﻬﻣ ﻡﻼﻫ ﻞﻜﺷ ﻰﻠﻋ ﺮﻀﺣ % 2 ﻝﻭﺯﺍﺪﻨﻴﺗ<br />
ﺓﻮﻗﻭ ﺔﺟﻭﺰﻠﻟﺍﻭ ﻲﻨﻴﺟﻭﺭﺪﻴﻬﻟﺍ ﺱﻻﺍ ﺱﺎﻴﻗ ﻢﺗ ﺎﻤﻛ<br />
. ﺎﻬﻟ ﺥﺎﻔﺘﻧﻻﺍ ﺔﺒﺴﻧ ﺱﺎﻴﻗ ﻢﺗ ﻲﺘﻟﺍﻭﺯﻮﻠﻴﻠﻴﺳ ﻞﻴﺜﻣ ﻲﺴﻛﻮﺑﺭﺎﻜﻟﺍﻭ ﺯﻮﻠﻴﻠﻴﺳ ﻞﻴﺜﻤﻟﺍ,<br />
ﺯﻮﻠﻴﻠﻴﺳ<br />
ﺕﺮﻬﻇﺍ ﺪﻗﻭ.<br />
4.2 ﻲﻨﻴﺟﻭﺭﺪﻴﻫ ﺱﺎﺑ ﻲﻠﺒﻬﻤﻟﺍ ﻂﻴﺤﻤﻠﻟ ﻪﺑﺎﺸﻤﻟﺍ ﻂﺳﻮﻟﺍ ﻲﻓ ﺓﺮﻀﺤﻤﻟﺍ ﺔﻴﻣﻼﻬﻟ ﺍ ﺐﻴﻛﺍﺮﺘﻟﺍ ﻦﻣ ءﺍﻭﺪﻟﺍ ﺭﺮﺤﺗﻭ ﻕﺎﺼﺘﻟﻻﺍ<br />
ﻕﺎﺼﺘﻟﺍ ﻰﻠﻋﺍﻭ ﺔﺟﻭﺰﻟ ﻰﻠﻋﺍ ﻰﻄﻋﺍ ﺯﻮﻠﻴﻠﻴﺳ<br />
ﻞﻴﺜﻣ ﻲﺴﻛﻮﺑﺭﺎﻛ % 3 ﻥﺃﻭ,<br />
ﻡﺪﺨﺘﺴﻤﻟﺍ ﺮﻤﻴﻟﻮﺒﻟﺍ ﺰﻴﻛﺮﺗﻭ ﻉﻮﻨﺑ ﺮﺛﺎﺘﻳ ءﺍﻭﺪﻟﺍ ﺭﺮﺤﺗ ﻥﺍ ﺞﺋﺎﺘﻨﻟﺍ<br />
ﻲﻓ ﺥﺎﻔﺘﻧﺍ ﺔﺒﺴﻧ ﻯﺍ ﻲﻄﻌﻳ ﻢﻟﻭ ءﺍﻭﺪﻠﻟ ﺭﺮﺤﺗ ﻰﻠﻋﺍ ﻰﻄﻋﺍ ﺪﻘﻓ ﺯﻮﻠﻴﻠﻴﺳ ﻞﻴﺜﻣ ﻞﻴﺑﻭﺮﺑ ﻲﺴﻛﻭﺭﺪﻳﺎﻬﻟﺍ ﺎﻣﺍ . ﺭﺮﺤﺗ ﺎﻄﺑﺍﻭ ﺥﺎﻔﺘﻧﺍ ﺔﺒﺴﻧ ﻰﻠﻋﺍﻭ<br />
Introduction<br />
The vaginal route of administration has<br />
many advantages compared to other routes (1).<br />
The vaginal epithelium is permeable to a wide<br />
range of drugs like hormones, peptides,<br />
proteins and antimycotics agents that need to<br />
reside at the site of infection for a prolonged<br />
period to be more effective. The vagina<br />
provides a promising site of systemic drug<br />
delivery because of its large surface area<br />
and its rich blood supply. In addition , a<br />
prolonged contact of a delivery system with<br />
the vaginal mucosa may be achieved<br />
more easily than at other route of drug<br />
absorption site like rectum or intestinal<br />
mucosa (2). . Tinidazole ( TND ) is {1- [ 2-<br />
(ethylsulphonyl) ethyl ] – 2 – methyl – 5 -<br />
nitroimidazole} has been proved to be very<br />
effective for the therapy of amoebiasis ,<br />
trichomonas , lambliasis and anaerobic<br />
infections (3) .The drug is well tolerated and<br />
widely used in clinical practice in the form of<br />
I.V infusion , tablets and vaginal pessaries (4).<br />
The efficacy of TND in the treatment of<br />
trichomonas vaginalis has been well<br />
documented (5) . TND is similar to<br />
metronidazole intravaginal gel can be used<br />
to treat bacterial vaginosis (6,7), it is effective<br />
, safe , easy to apply and has fewer side<br />
effect compared to oral dosage forms of<br />
1Corresponding author E- mail : Zainabthabit@yahoo.com<br />
Received : 3/5/2010<br />
Accepted : 18/9/2010<br />
64<br />
. ﻡﺪﺨﺘﺴﻤﻟﺍ ﺰﻴﻛﺮﺘﻟﺍ<br />
this medication (8,9) patients tolerate gels<br />
better than vaginal inserts or ointments. (10)<br />
This study was conducted to formulate TND in<br />
a suitable mucoadhesive vaginal gel through<br />
studying different variables affecting the<br />
physicochemical properties and the in vitro<br />
release of the drug from these preparations.<br />
Materials and Methods<br />
Materials<br />
Tinidazole ( TND ) was supplied by<br />
Sigma chemical co., methylcellulose( MC)<br />
and carboxymethylcellulose(CMC)from(BDH<br />
limite ( HPMC) from ( Shin-Etsu chemical<br />
co.– Germany) . All other materials used were<br />
of analytical grade.<br />
Methods<br />
Preparation of simulating vaginal fluid<br />
(S.V.F)<br />
Simulating vaginal fluid (S.V.F ) was<br />
prepared by dissolving 3.51gm of sodium<br />
chloride ,1.4gm of potassium hydroxide<br />
,0.22gm of calcium hydroxide , 0.018gm of<br />
bovine serum albumin, 2 gm of lactic acid ,<br />
1gm of acetic acid ,0.16gm of glycerol<br />
,0.4gm of urea and 5gm of glucose in 1 liter<br />
distilled water then the pH was adjusted at<br />
4.2 (11).
Iraqi J Pharm Sci, Vol.19(2) 2010 Tinidazole bioadhesive vaginal gels<br />
Preparation of TND vaginal aqueous gel<br />
bases<br />
The tested aqueous gel bases were<br />
prepared by dispersing the polymers<br />
powder of 3 and 4%(w/w) MC ,2 and<br />
3%(w/w) CMC and 3 and 4%(w/w) HPMC in<br />
cold freshly distilled water with the aid of a<br />
high speed stirrer , until a solutions were<br />
complete (12) .The products were refrigerated<br />
for 1hour before use to obtain a suitable<br />
consistency .TND gels were prepared by<br />
dispersing 2% drug in different gel bases as<br />
shown in table(1).<br />
Table 1:Composition of TND bioadhe sive<br />
vaginal ge l<br />
In vitro dissolution of TND from gel bases<br />
The release study was carried out in a<br />
USP apparatus type І at 100 rpm ,a basket<br />
of 2.5 cm in diameter was enclosed with a<br />
multifold filter paper (dialysis cell) in order to<br />
be filled with 1gm of each base containing<br />
2%w/w TND. After connecting to a stirrer<br />
motor , the dialysis cell was immersed to<br />
about 1 cm of its surface in 500 ml of<br />
simulating vaginal fluid pH4.2 at 37 ◦ C for 5<br />
hours .Samples were withdrawn after<br />
0.5,1,2,3,4 and 5 hours and replaced with<br />
an equal volume of the same fluid solution .<br />
The samples were analyzed for their drug<br />
content at its λmax 320nm (13, 14). .<br />
The effect of polymer type on the release of<br />
TND from the gel bases<br />
The effect of MC, CMC and HPMC<br />
bases on the release of TND from gel bases<br />
was studied.<br />
The effect of polymer concentration on<br />
the release of T ND from the gel bases<br />
The effect of 3 and 4% w/w MC , 2<br />
and 3% w/w CMC and 3 and 4% w/w<br />
HPMC on the release of TND from gel<br />
bases were studied.<br />
Measurement of the swelling index of dry<br />
polymer in S.V.F<br />
The swelling index is the volume in<br />
ml occupied by 1 gm of polymer after it<br />
has swollen in aqueous liquid (S.V.F used<br />
in the study) for four hours. The process is<br />
carried out by placing 1gm of dry polymer<br />
in 25ml graduated cylinder , moisten it with<br />
1ml (ethanol 96%) and with an addition of 25<br />
ml S.V.F shaking vigorously every 10<br />
minute for one hour ,then allowing to stand<br />
for three hours . After1.5houre from<br />
beginning the test any volume of liquid<br />
retained in the layer of the polymer and any<br />
particles of polymer floated at the surface<br />
of the liquid were released (15) .The swelling<br />
was measured according to this equation:<br />
Swelling =<br />
(final volume- initial volume)<br />
initial volume<br />
Mechanism of TND release from gel bases<br />
To understand the mechanism of the<br />
drug release , the release profiles from<br />
different gel bases, the % fraction drug<br />
released verses time profiles were plotted<br />
using linear fitted equations proposed by<br />
Koresmeyer - Peppas<br />
( 16 )<br />
F = Mt/Mo = ktⁿ at time t (1)<br />
ln F = ln k + n ln t (2)<br />
The equations express the fraction (F) release<br />
of drug from gel Where Mt is the amount<br />
released at time t ,Mo is the initial amount of<br />
drug ,K is the rate constant and n is the<br />
releasing exponent value indicative the<br />
mechanism of drug release( if n is 0.5 or<br />
less (fickian ) holds the release, while<br />
when n greater than 0.5 this indicate nonfickian<br />
(anomalous) releasing mechanism),<br />
n value could be result from the slope<br />
obtained by the drawing of ln. Fraction<br />
released verses ln. Time.<br />
Viscosity measurement<br />
A Brook Field digital viscometer with a<br />
suitable sample adaptor was used to measure<br />
the viscosity in centipoises of the bioadhesive<br />
prepared gel. (17)<br />
Determination of pH<br />
The pH of the bioadhesive gels were<br />
determined by digital pH meter .One gram of<br />
gel was dissolved in 25 ml of dislilled water<br />
and the electrode dipped into gel formulation<br />
for 30 minute..The measurements of pH of<br />
each system were replicated three times (18)
Iraqi J Pharm Sci, Vol.19(2) 2010 Tinidazole bioadhesive vaginal gels<br />
Determination of mucoadhesive force<br />
The mucoadhesive potential of each<br />
system was determined by measuring the force<br />
required to detach the gel from sheep vaginal<br />
mucosal tissue by using a modified chemical<br />
balance. Asection of vaginal mucosa was cut<br />
from the sheeps vaginal cavity and instantly<br />
fixed with mucosal side out onto each glass<br />
vial using a rubber band. The vials with<br />
vaginal mucosa were stored at 37 ◦ C for 5<br />
minutes .Then next vial with a section of<br />
mucosa was connected to the balance in<br />
inverted position, while first vial was placed<br />
on a height adjustable pan. Fixed amount of<br />
sample of each gel system were placed onto<br />
the vaginal mucosa of first vial. Then the<br />
height of second vial was adjusted so that<br />
mucosal surfaces of both vials come in<br />
intimate contact .Five minutes contact time<br />
was given to ensure intimate contact between<br />
tissues and the sample. Then weight was kept<br />
rising in the pan until vials get detached. The<br />
bioadhesive forces as the detachment stress in<br />
dyne/cm 2 , was determined from the minimal<br />
weights that detached the tissues from the<br />
surface of each gel using the following<br />
equation (19):<br />
Detachment stress(dyne/cm 2 )= m g/A<br />
Where, m= weight required for detachment of<br />
two vials in grams.<br />
g= Acceleration due to gravity [980cm/s 2 ].<br />
A=area of tissue exposed.<br />
The vaginal mucosa was changed for each<br />
measurement .Measurements were repeated<br />
three times for each of the gel system.<br />
Results and Discussion<br />
In order to fortify the adhesion of<br />
administered drug onto the mucosal<br />
surface, TND was formulated in a suitable<br />
mucoadhesive polymers , MC, CMC and<br />
HPMC bases to take a full advantageous of<br />
66<br />
the contact time , the drug can be<br />
dispersed in the gel giving a concentration<br />
that is higher than corresponding to the<br />
solubility of the drug (20) ,as well as ,it can be<br />
considered as a way for sustaining the release<br />
of the drugs from gel dosage form . MC, CMC<br />
and HPMC were used as a gel base<br />
because of there safety use for vaginal<br />
application as well as there compatibility with<br />
TND, with other vaginal secretion.<br />
The effect of polymer type and concentration<br />
on the releasing of TND from the gel bases<br />
Figures 1, 2 and 3 showed the amount<br />
of TND released from gel bases in<br />
S.V.F medium .It was found that the amount<br />
of TND released from gel bases were<br />
significantly influenced (p
F fraction released<br />
F fraction released<br />
Iraqi J Pharm Sci, Vol.19(2) 2010 Tinidazole bioadhesive vaginal gels<br />
Figure 1 : The effect of MC Concentration<br />
on the release profile of TND at pH 4.2 and<br />
37 time(minute)<br />
C<br />
Figure 2: The effect of CMC on the release<br />
profile of TND at pH 4.2 and 37 C<br />
F fraction released<br />
0.4<br />
0.35<br />
0.3<br />
0.25<br />
0.2<br />
0.15<br />
0.1<br />
0.05<br />
0.4<br />
0.35<br />
0.3<br />
0.25<br />
0.2<br />
0.15<br />
0.1<br />
0.05<br />
0<br />
0<br />
0 100 200 300 400<br />
0.3<br />
0.25<br />
0.2<br />
0.15<br />
0.1<br />
0.05<br />
2% w/w CMC<br />
3% w/w CMC<br />
0 100 200 300 400<br />
time(minute)<br />
0<br />
3% w/w HPMC<br />
4% w/w HPMC<br />
3% w/w MC<br />
4% w/w MC<br />
0 100 200 300 400<br />
time(minute)<br />
Figure 3 : The effect of HPMC on the<br />
release profile of TND at pH 4.2 and 37 C<br />
67<br />
Figure 4 : The swe lling mechanism of 3 &<br />
4% w/w MC in simulating vaginal fluid pH<br />
4.2 and 37 C<br />
t/v (hr/ml fluid solution /ml CMC )<br />
3.5<br />
3<br />
2.5<br />
2<br />
1.5<br />
1<br />
0.5<br />
0<br />
3% CMC<br />
2% CMC<br />
1 2 3 4 5<br />
time(hr)<br />
Figure 5 : The swelling mechanism of 2 &<br />
3% w/w C MC in simulating vaginal fluid<br />
pH 4.2 and 37 C<br />
Releasing mechanism of TND from gel bases<br />
It was found that formulation with higher<br />
swelling index retard the release of drugs more<br />
than those with lower swelling indices , this<br />
swelling also depends on the pH of the<br />
medium and the presence of electrolytes ,<br />
where the S.V.F pH 4.2 has a lot of<br />
electrolytes content such as : sodium chloride ,<br />
potassium hydroxide , calcium hydroxide (25).<br />
This swelling is important since its<br />
significantly decreases the releasing rate of the<br />
drug and increases in the amount of the drug<br />
release after five hours ,as shown in table-3-<br />
.The reason behind this increasing in the<br />
amount released due to erosion of the gel as a<br />
result of severe hydration or relaxation of the<br />
polymer , so interchain intermolecular force<br />
will no longer be able to resist any external<br />
forces ,once gel erodes ,it breaks up into<br />
smaller and smaller particles ,more surfaces<br />
will be exposed to the fresh swelling medium
Iraqi J Pharm Sci, Vol.19(2) 2010 Tinidazole bioadhesive vaginal gels<br />
and hence more drug will be released leads to<br />
an increasing in the amount of the drug<br />
released from CMC gel base more than other<br />
swollen MC gel base after five hours (18) .As a<br />
result , CMC gel bases hold a Fikian release<br />
mechanism.<br />
Table 3 :The release rate, amount released and the releasing mechanism of the TND from gel bases<br />
Polymer type<br />
and<br />
concentration<br />
MC 3%<br />
MC 4%<br />
CMC 2%<br />
CMC 3%<br />
HPMC 3%<br />
HPMC 4%<br />
n expone nt<br />
release value<br />
0.41<br />
0.49<br />
0.509<br />
0.52<br />
0.29<br />
0.5<br />
Viscosity measurment<br />
Viscosity is an important parameter for<br />
characterizing the gel as it affects the<br />
spreadibility ,extrudability and release of<br />
drug (26) .CMC gel base show the highest<br />
viscosity, and as the polymer concentration<br />
increase ,the viscosity will be increased as<br />
shown in table 4. On the other hand , minimum<br />
level of viscosity was seen with F5 and F6<br />
explain the rapid release of drug fro m this gel<br />
system and this due to non swelling property<br />
of HPMC polymer at these concentration .<br />
Table 4:The viscosity and pH of the<br />
prepared TND bioadhe sive vaginal gel<br />
Formula Viscosity pH<br />
No. (cps)<br />
F1 1450 6.02<br />
F2 3240 5.2<br />
F3 1700 5.03<br />
F4 6800 5.17<br />
F5 14.5 5.02<br />
F6 21.6 5.2<br />
Determination of pH<br />
The pH of gels were found to be within<br />
the range of 5-6.2 which is within the limit of<br />
semisolid specification and at this pH the gel<br />
will be non irritant to vagina .(12). The physical<br />
appearance of the gel was evaluated with<br />
naked eye . The gel was smooth without any<br />
lumps and of uniform color ,no color change<br />
/liquification /separation were observed .<br />
Determination of mucoadhesive force<br />
Scheme 1 indicates the vaginal bioadhesive<br />
properties of the prepared gels in sheep<br />
vagina,and the resultes showed that all vaginal<br />
bioadhesive strengths were found in the<br />
K % minⁿ<br />
re leasing<br />
rate<br />
5.3974<br />
3.5744<br />
4.1594<br />
3.582<br />
6.1472<br />
3.1864<br />
amount re le ase<br />
(mg/5hr)<br />
(mean±SD,n=3)<br />
7.05±0.14<br />
4.466±0.13<br />
7 ±0.15<br />
4.35±0.12<br />
5.2±0.03<br />
4.58±0.11<br />
Mechanism of<br />
TND release<br />
Fickian<br />
Fickian<br />
Fickian<br />
Fickian<br />
Fickian<br />
Fickian<br />
following order F2,F4>F3>F1>F6>F5<br />
Indicating that (F2)and (F4) showed the<br />
highest bioadhesive properties.<br />
Sche me 1: Bioadhesive stre ngth of TND<br />
Bioadhesive vaginal gels<br />
Conclusion<br />
Results of this study confirm that, the<br />
physical properties of the prepared gel were<br />
affected by the type and concentration of the<br />
polymer used in the preparation. The more<br />
sustained preparation with highest bioadhesive<br />
force and highest viscosity was obtained by the<br />
use of 3% CMC as a gel base.In addition to<br />
that the mechanism of drug release from<br />
tinidazole 3%CMCgel base was diffusion<br />
mixed with erosion.<br />
References<br />
1. Richardson JI Illum L The vaginal route<br />
of peptide & protein drug delivery .Adv.<br />
drug delivery rev 1992 ; 8: 341-366 .<br />
2. Claudia, KV, Constantiae, Irene H. ,<br />
Andreas, SB . Development and in vitro<br />
evaluation of a mucoadhesive vaginal<br />
delivery system for progesterone. J.<br />
controlled release 2001; 77: 323-332.
Iraqi J Pharm Sci, Vol.19(2) 2010 Tinidazole bioadhesive vaginal gels<br />
3. Trac JW. Webster LT. Drugs used in<br />
the chemotherapy of protozoal<br />
infection .In:Hardman, J.,G., Limbird,L.<br />
,E.,the pharmacological basis of<br />
therapeutics 9 th ed. Mc Graw –Hill,New<br />
York 1996 ; 995-998<br />
4. Martindale. The complete drug<br />
reference, 32 ed., pharmaceutical press<br />
1999; 594: 1099, 1541.<br />
5. BNF, British national formulary, British<br />
medical association and Royal<br />
Pharmaceutical society of Great Britain<br />
2004; 47 ed.: 287, U.K.<br />
6. Milani,M., Barcellona,E., Agnello,A.,<br />
Efficacy of the combination of 2g oral<br />
tinidazole and acidic buffering vaginal<br />
gel in comparison with vaginal<br />
clindamycin alone in bacterial vaginosis<br />
:a randomized ,investigator -blinded,<br />
controlled trial .Euro .J. of obst. and gyne<br />
2003 ;109: 67-71.<br />
7. Livengood, . G. H. Mc Gregor , ., J.,A.,<br />
Soper, D., E, Newton, E., Thomason, J.,L.,<br />
Bacterial vaginosis : efficacy and<br />
safety of intravaginal metronidazole<br />
treatment .Am.J.Obstet.Gynecol 1994<br />
;170: 759-764.<br />
8. Hanson, JM., McGregor, JA, Hiller,<br />
SL., Eschenbach, DA., Kreutner A.K.,<br />
Galask, RP., Martens, M..<br />
Metronidazole for bacterial vaginosis.<br />
Acomparision of vaginal gel vs. oral<br />
therapy .J.Reprod.Med 2000; 45: 889-896.<br />
9. Dubonchet, L. , McGregor, J.A. , Is mail<br />
, M. , McCormack, W.M. , A pilot study<br />
of metronidazole vaginal versus oral<br />
metronidazole for tretmeant of<br />
trichomonas vaginalisvaginitis ,sex.<br />
Transm. di 1998 ;25:176-179.<br />
10. Edsman, K. Carlfors J. Peterson R.<br />
Reological evaluation of poloxamer as<br />
an in situ gel for ophthalmic use.<br />
Eur.J.Pharm.Sci. 1998; 6: 105-112.<br />
11. Owen,DH., Peters JJ., Lavine L.L., Katz<br />
D.F., Effect of temperature and pH on<br />
the contraceptive gel viscosity<br />
.Contraceptive 2003;67: 57-64 .<br />
12. Farhan, J.A , Mohd ,A .A., Zeenat .I K ,<br />
Roop, K. K. and Mushir, A . Development<br />
and in vitro evaluation of an acid<br />
buffering bioadhesive vaginal gel for<br />
mixed vaginal infection . Acta pharm.<br />
2008 ; 58: 407-419 .<br />
13. Mariee, N.K. Factors affecting the in<br />
vitro diffusion of diclofenac sodium<br />
from ointment. Iraqi .J. of pharmacy<br />
2001; 1: 62-71<br />
14. Hui, H.W., Robinson, J.R., Effect of<br />
particle dissolution rate on ocular<br />
69<br />
drugbioavailability .J. pharma. sci. 1986;<br />
75: 280-287.<br />
15. BP ,British Pharmacopoeia 2008 ;vol. IV,<br />
Appendix XI E A273.<br />
16. Martin A.N. “Physical Pharmacy”, 4 th ed ,<br />
Waverly, New Delhi. 1993; 497.<br />
17. Ritger, PL, Peppas, NA. Modelling of<br />
water transport solute release in<br />
physiologically sensitive gels. J.control<br />
release 1987; 5:37-40 .<br />
18. Benoy, B.B.,Bhalani, S.N. and Arkenduc .<br />
Formulation development and<br />
characterization of metronidazole<br />
microencapsulated bioadhesive vaginal<br />
gel. Int. J. of pharmacy and<br />
pharmaceutical sciences 2009; 1, Issue1.<br />
19. Yong, C S, Choi, T S, Quan, Q Z . Rhee,<br />
J D, Kim, CK , Lim, S J, Effect of Na<br />
chloride on the gelation temperature,gel<br />
strength and bioadhsive force of<br />
poloxamer gels containing diclofenac Na.<br />
Int. J .pharm. 2001; 226:195-205.<br />
20. Veyries ,M.,L., Couarraze,G., Geiger,S.,<br />
Agnely,F., Massias,L., Kunzli,B.,<br />
Faurisson,R., Rouveix,B. ,<br />
Controlled release of vancomycin<br />
from poloxamer 407 gels<br />
.Int.J.Pharm. 1999; 192: 183-193.<br />
21. Mirchandan HL ,Chien YW ,Bruce PD,<br />
Senshang L ,Effect of HPMC and<br />
carbopol on the release and floating<br />
properties of gastric floating drug delivery<br />
system using factorial design Int.J.Pharm.<br />
2003; 253: 3-22.<br />
22. Jaleh, V, Nasser T., Sharona ,S<br />
.,Development and physical<br />
characterization of a periodontal<br />
bioadhesive gel of metronidazole, Drug<br />
deliv.2002; 9, issue 2: 127-133.<br />
23. Martinez, N Vazquez, Antoniocruz, R .del<br />
C ,Alvarez Castillo A ,Mendoza, A M ,<br />
Martinez, A B, Morales Cepeda ,<br />
Swelling kinetic of hydrogels from methyl<br />
cellulose and poly acrylamide .I S S N<br />
2007 ; 6 : 337-345.<br />
24. Naim, S., Samuel, B., Chauhan, B.,<br />
Paradkar, A. Effect of potassium chloride<br />
and cationic drug on swelling, erosion and<br />
release from κ-carrageenan matrices.<br />
AAPS PharmSciTech. 2004; 5(2): article<br />
25.<br />
25. Omidian, H, Park, k., Swelling agents and<br />
devices in oral drug delivery ,J.drug<br />
.del.sci.tech. 2008; 18(2): 83-93.<br />
26. Sanap, G.S., Smita, T., Tarannum, S. and<br />
Sangita, G. Formulation and evaluation of<br />
mucoadhesive beads of glipizide by 2 3<br />
factorial design .jou. of phar. Res. 2009;<br />
2(5): 934- 938.
Iraqi J Pharm Sci, Vol.19(2) 2010 Protein profiles in children with Kala-azar<br />
Urine Protein SDS-PAGE Reveals Different Profiles in Iraqi<br />
Children with Kala-azar<br />
Yassir MK Al-Mulla Hummadi* ,1<br />
* Department of Pharmaco-therapeutics, College of Pharmacy, Al-Mustansiriyah University, Baghdad, Iraq.<br />
Abstract<br />
Urine proteomics have been an area of interest and recently in Kala-azar as an alternative sample<br />
type for serum or plasma. Because of simplicity, noninvasiveness of collection and simpler matrix.<br />
Many studies had detected an increased protein excretion in the urine of patients with active Kala-azar<br />
due to renal involvement particularly by an immunological related mechanism(s). This study have<br />
demonstrated the presence of three different protein profiles in Iraqi children (Patients: including 60<br />
children aged 4-60 months) with defined Kala-azar using the conventional SDS-PAGE on urine<br />
samples. Urine protein profile in Kala-azar patients revealed three groups of banding patterns: group-<br />
1(33.4)% of the patients show the pattern of 5 bands with a MW. Ranged (512.861-158.489), groups-2,<br />
3 were (55, 11.6) % of the patients showing 2 banding proteins with a MW. ranged (512.861-199.526) ,<br />
(199.526-181.97) respectively. These findings may be correlated with other epidemiological studies<br />
that revealed the presence of different clinical presentations like fever, spleenomegally, hepatomegally,<br />
thro mbocytopaenia, and different response to leishmania therapy. Furthermore, the presence of<br />
different protein patterns may also be related to the chronicity of infection and the degree of renal<br />
involvement. The presence of a similar protein band between group-1 and 2 may be of diagnostic<br />
purpose and further studies on expanded number of patients are required to identify that kind of protein<br />
or other urine protein profiles.<br />
Key words: Protein band, prote in pattern, sodium dode cyl sulphate -poly acrylamide gel<br />
electrophoresis (SDS-PAGE), urine proteome, visceral leishmaniasis.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﻭَﺃ ِﻞﺼﻤﻟﺍ ﺕﺎﻨﻴﻌﻟ ﻞﻳﺪﺑ ﺝﺫﻮﻤﻨﻛ ﻪﻴﺋﺎﺸﺣﻻﺍ ﺕﺎﻴﻧﺎﻤﺸﻠﻟﺍ ءﺍﺩ ﻲﻓ ًﺍﺮﺧﺆﻣﻭ ﻡﺎﻤﺘﻫﺍ ﺓﺮﺋﺍﺩ<br />
ﻝﻮﺒﻟﺍ ﺕﺎﻨﻴﻋ ﻲﻓ ﺕﺎﻨﻴﺗﻭﺮﺒﻟﺍ ﻞﻜﺸﺗ<br />
ﻪﺗﺩﺎﻣ ﺔﻃﺎﺴﺑ ﻰﻟﺍ ﻪﻓﺎﺿﺍ ﻪﺣﺭﺎﺠﻟﺍ ﺕﺍﻭﺩﻻﺎﻛ ﻪﻌﺿﺎﺒﻟﺍ ﻞﺋﺎﺳﻮﻟﺍ ﺐﻨﺠﺘﺑ ﻪﻌﻤﺟ ﺔﻟﻮﻬﺳﻭ ﻪﻴﻠﻋ ﻝﻮﺼﺤﻟﺍ ﻲﻓ ِﺔﻃﺎﺴﺒﻟﺍ ﺐﺒﺴﺑ . ﺎﻣﺯﻼﺒﻟﺍ<br />
ﺽﺮﻤﻠﻟ ﺔﻄﻴﺸﻨﻟﺍ ﻩﺮﺘﻔﻟﺍ ﻲﻓ ﻪﻴﺋﺎﺸﺣﻻﺍ ﺎﻴﻧﺎﻤﺸﻠﻟﺎﺑ ﻦﻴﺑﺎﺼﻤﻟﺍ<br />
ﻰﺿﺮﻤﻟﺍ ِﻝﻮﺑ ﻲﻓ ﺪﻳﺍﺰﺘﻣ ِﻦﻴﺗﻭﺮﺑ َﺡﺮﻃ ِﺕﺎﺳﺍﺭِﺪﻟﺍ ْﻦِﻣ ﺪﻳﺪﻌﻟﺍ ْﺖﻔﺸﺘﻛﺇ . ﻪﻴﺳﺎﺳﻻﺍ<br />
ﻝﻮﺑ ﺝﺫﺎﻤﻧ ﻲﻓ ِﺔﻔﻠﺘﺨﻣ ِﻦﻴﺗﻭﺮﺑ ﻁﺎﻤﻧﺍ ﻊﻴﻣﺎﺠﻣ ﺙﻼﺛ َﺩﻮﺟﻭ ِﺔﺳﺍﺭﺪﻟﺍ ﻩﺬﻫ ﺖﻔﺸﻛ . ﻪﻴﻋﺎﻨﻤﻟﺍ ِﺔﻴﻟﻵﺎﺑ ﻖﻠﻌﺘﻳ ﺎﻤﺑ ًﺎﺻﻮﺼﺧ ِﻱﻮﻠﻜﻟﺍﺮﺛﺎﺘﻟﺍ ﺐﺒﺴﺑ<br />
ﻲﺋﺎﺑﺮﻬﻜﻟﺍ ﻞﻴﺣﺮﺘﻟﺍ ﺔﻴﻨﻘﺗ ﻡﺍﺪﺨﺘﺳﺎﺑ ﻚﻟﺫﻭ ( ﺮﻬﺷ 604<br />
ِﺮﻤﻌﺑ ﻞﻔﻃ 60 : ﻪﻴﺋﺎﺸﺣﻻﺍ ﺎﻴﻧﺎﻤﺸﻠﻟﺎﺑ ﻦﻴﺼﺨﺸﻤﻟﺍ ﻰﺿﺮﻤﻟﺍ)<br />
ِﻦﻴﻴﻗﺍﺮﻌﻟﺍ ِﻝﺎﻔﻃﻷﺍ<br />
ﻡﺰﺣ ﺲﻤﺧ ﺕﺮﻬﻇﺍ ﻰﺿﺮﻤﻟﺍ ﻦﻣ % ( 33.4)<br />
1 ﺔﻋﻮﻤﺠﻣ : ﻲﻠﻳ ﺎﻤﻛ ﺖﻧﺎﻛ ﺪﻗﻭ . ِﻝﻮﺒﻟﺍ ِﺕﺎﻨﻴﻋ ﻰﻠﻋ ( ﺪﻳﺎﻣﺍ ﻞﻳﺮﻛﻻﺍ ﺩﺪﻌﺘﻣ ﻡﻼﻫ)<br />
ﻱﺪﻴﻠﻘﺘﻟﺍ<br />
ﺕﺮﻬﻇﺍ ﻰﺿﺮﻤﻟﺍ ﻦﻣ % ( 11.6 ٬ 55)<br />
ْﺖﻧﺎَﻛ 3 ﻭ 2 ﻪﻋﻮﻤﺠﻣ ٬ﻥﻮﺘﻟﺍﺩ ( 158.489<br />
512.861 ) ﻦﻴﺑ َﺡﻭﺍﺮَﺗ ﻲﺌﻳﺰﺟ ﻥﺯﻭ ﻊَﻣ ﻪﻴﻨﻴﺗﻭﺮﺑ<br />
. ﻲﻟﺍﻮﺘﻟﺍ ﻰﻠﻋ ﻭ ﻥﻮﺘﻟﺍﺩ ( 181.97<br />
199.526)<br />
٬ ( 199.526 512.861<br />
) ﻦﻴﺑ َﺡﻭﺍﺮَﺗ ﻲﺌﻳﺰﺟ ﻥﺯﻭ ﻊَﻣ ﺎﻤﻬﻨﻣ ﻞﻜﻟ ِﻦﻴﺗﻭﺮﺒﻟﺍ ﻦﻣ ﻦﻴﺘﻣﺰﺣ<br />
ﺪﺒﻜﻟﺍ ﻢﺨﻀﺗ<br />
‘ ﻰﱠﻤُﺤﻟﺍ ﻞﺜﻣ ِﺔﻔﻠﺘﺨﻤﻟﺍ ِﺔﻳﺮﻳﺮﺴﻟﺍ ِﺽﺭﺍﻮﻌﻟﺍ ﺩﻮﺟﻭ ْﺖﻔﺸَﻛ ﻲﺘﻟﺍﻭ ﻪﻘﺑﺎﺴﻟﺍ ﻯﺮﺧﻷﺍ ﻪﻴﺋﺎﺑﻮﻟﺍ ِﺕﺎﺳﺍﺭِﺪﻟﺎﺑ ُﻂَﺒْﺗﺮُﺗ ْﺪَﻗ ِﺞﺋﺎﺘﻨﻟﺍ ﻩﺬﻫ<br />
ًﺎﻀﻳﺃ ﺎَﻤﱠﺑُﺮَﻟ ِﺔﻔﻠﺘﺨﻤﻟﺍ ِﻦﻴﺗﻭﺮﺒﻟﺍ ِﻁﺎﻤﻧﺃ ﺭﻮﻀﺣ ٬ﻚﻟﺫ ﻰﻠﻋ ﺓﻭﻼﻋ . ﻪﻴﺋﺎﺸﺣﻻﺍ ﺎﻴﻧﺎﻤﺸﻠﻟﺍ ِﺝﻼﻌﻟ ﻪﻔﻠﺘﺨﻣ ّﺕﺎﺑﺎﺠﺘﺳﺍﻭ ٬ﺕﺎﺤﻴﻔﺼﻟﺍ ﺔﻠﻗ ٬ﻝﺎﺤﻄﻟﺍﻭ<br />
ﻥﺎﻜﻤﺑ ﺓﺪﺋﺎﻔﻟﺍ ْﻦِﻣ ﻥﺎﻧﻮُﻜَﺗ ْﺪَﻗ 2ﻭ<br />
1 ِﺔﻋﻮﻤﺠﻤﻟﺍ ﻦﻴﺑ ِﺔﻠﺛﺎﻤﻣ ِﻦﻴﺗﻭﺮﺑ ﺔﻣﺰﺣ ﺩﻮﺟﻭ ﻥﺇ ﺎﻤﻛ . ِﻱﻮﻠﻜﻟﺍ ِﻞّﺧﺪﺘﻟﺍ ِﺔﺟﺭﺩﻭ ﺽﺮﻤﻟﺍ ﻥﺎﻣﺯﺎﺑ ﺔﻘّﻠَﻌَﺘُﻣ<br />
ﻁﺎﻤﻧﺃ ﻒﺸﻛ ﻭَﺃ ِﻦﻴﺗﻭﺮﺒﻟﺍ ْﻦِﻣ ِﻉﻮﻨﻟﺍ ﻚﻟﺫ ﺰﻴﻴﻤَﺘﻟ ﻰﺿﺮﻤﻟﺍ ْﻦِﻣ ِﻊّﺳﻮﻣ ِﺩﺪﻋ ﻰﻠﻋﻭ ﻯﺮﺧﺃ ِﺕﺎﺳﺍﺭِﺩ ءﺍﺮﺟﺍ ﻲﻋﺪﺘﺴﻳ ﺎﻤﻣ ِﻲﺼﻴﺨﺸﺘﻟﺍ ِﺽﺮﻐﻠﻟ<br />
Introduction<br />
Visceral leishmaniasis and cutaneous<br />
leishmaniasis caused by Leishmania donovani<br />
and Leishmania tropica or Leishmania major<br />
respectively are known endemic diseases in<br />
Iraq.Visceral leishmaniasis had been prevalent<br />
in Iraq for the last 2-3 decades causing serious<br />
problems (1) . It represents an important factor<br />
in infant's mortality in this country<br />
.Diagnosis of visceral leishmaniasis generally<br />
based on clinical presentation and<br />
microscopical demonstration of the parasite in<br />
smears, aspirates of bone marrow or culture of<br />
these aspirates on certain culturing media.<br />
However, the techniques are painful,<br />
1Corresponding author E- mail : yassir.almullahummadi@ymail.com<br />
Received : 25/5/2010<br />
Accepted : 29/9/2010<br />
(2)<br />
70<br />
. ِﻝﻮﺒﻟﺍ ﺕﺎﻨﻴﻋ ﻲﻓ ﺕﺎِﻨﻴﺗﻭﺮﺒﻟﺍ ﻦﻣ ﻯﺮﺧﺃ<br />
hazardous and need skilled personnel and<br />
equipped hospitals some times difficult to be<br />
obtained (3) . Since visceral leishmaniasis is<br />
occurring in places of poor socioeconomic<br />
conditions where health services are poorly<br />
developed (4) , the diagnosis in this case is<br />
difficult to be obtained using these<br />
techniques (3) . However, presence of<br />
Leishmania donovani soluble antigen,<br />
corresponding antibody and component of the<br />
complement in the serum of patient with active<br />
kala-azar has been demonstrated in a number<br />
of studies (1) , Al-Bashir (5) .
Iraqi J Pharm Sci, Vol.19(2) 2010 Protein profiles in children with Kala-azar<br />
On the other hand, the detection of Leishmania<br />
donovani parasite antigen in the urine samples<br />
of patient with active Kala-azar has been<br />
demonstrated in a number of studies by<br />
(6)<br />
immunoblotting ; enzyme linked<br />
(7)<br />
immunosorbent assay (ELISA) ; latex<br />
agglutination test (LAT)<br />
(8) and direct<br />
agglutination test (DAT) (8, 9) .Furthermore,<br />
urine samples are more easily to be collected<br />
especially from young children, as well as<br />
facilitating field studies (9) .Therefore, this<br />
study aims to demonstrate the presence of<br />
variations in protein profiles in a number of<br />
Iraqi patients with defined visceral<br />
leishmaniasis using the conventional sodium<br />
dodecyl sulphate-poly acrylamide gel<br />
electrophoresis (SDS-PAGE) in urine samples.<br />
Patients and Methods<br />
This study was divided into two groups:<br />
1. Patients: including 60 children aged 4-60<br />
months, admitted to paediatric ward of Alkadhimiyah<br />
teaching hospital-Al-Nahrain<br />
University from December 2007-<br />
Febriwary 2009. All were proven cases of<br />
Kala-azar by: clinical manifestation of<br />
fever, hepato-spleenomegally, the<br />
demonstration of the parasite - the<br />
amastigotes from indirect smear of bone<br />
marrow and serological diagnosis by<br />
indirect immunofluorescence antibody test<br />
IFAT.<br />
2. Control: consist from 10 healthy children<br />
all were seronegative.<br />
Urine samples<br />
24 hours urine specimens were collected<br />
from each child in the control or the diseased<br />
group. Urine samples from all children were<br />
concentrated 100 times using Amicon<br />
Apparatus (Amicon Corporation Davers, MA)<br />
and kept at -20 o C till use to avoid bacterial<br />
contamination as described by Baqir et al.<br />
(2002) (6) ; Al-Bashir and Ali (2003) (8) .Total<br />
protein for each sample was measured<br />
according to the method of Lowery et al.<br />
(1951) (10) . The protein electrophoresis was<br />
dependent on the conventional polyacrylamid<br />
gel electrophoresis PAGE as described by<br />
Fehrnstrom and Morberg (1977) (11) .<br />
Urine protein profile<br />
Urine protein electrophoresis was<br />
performed as described by Fehrnstrom and<br />
Morberg (1977) (11) , using Coomaise brilliant<br />
blue staining.Migration distance of the<br />
calibration proteins were measured after<br />
staining the protein bands. The relative<br />
mobility (RM) was measured too according to<br />
the following equation:<br />
71<br />
The calibration curve was constructed by<br />
plotting RMs versus log. Molecular weights<br />
for calibration proteins Fig.(1). The molecular<br />
weight of proteins of interest was determined<br />
from the position of its RM value on the<br />
calibration curve.<br />
Figure 1: Standard curve for approximate<br />
estimation of molecular weights of various<br />
prote ins inurine samples.<br />
Results<br />
Conventional polyacrylamide gel<br />
electrophoresis has been used to differentiate<br />
between protein patterns in serum of normal<br />
and patients with Kala-azar. Coomassie<br />
brilliant blue stain has been used to visualize<br />
the band separated on the gel (12) . Urine of the<br />
control group has a specific protein pattern<br />
with one protein bands fig.(2).<br />
Control group (1) group (2) group (3)<br />
Figure 2 : Urine prote in profiles in normal<br />
and kala-azar children.<br />
The relative mobility value (RM) was 0.48<br />
with a molecular weight 199.525.Urine protein<br />
profile in Kala-azar patients revealed three<br />
banding patterns ranged from (2-5) protein
Iraqi J Pharm Sci, Vol.19(2) 2010 Protein profiles in children with Kala-azar<br />
bands. The (RM) values were ranged from<br />
(0.22-0.52). See fig.(2). The first protein<br />
pattern demonstrates five bands. The RM<br />
values were ranged from (0.22-0.52), with<br />
molecular weights ranged from (512.861 -<br />
158.489) ×10 3 Dalton respectively. Four out of<br />
these bands were shown to be abnormal from<br />
the control these are bands (1, 2, 3 and 5) as<br />
shown in table (1). This band picture was<br />
found in 20 patients (33.4 %) Table (2). The<br />
second protein pattern shows two bands the<br />
RM values were ranged from (0.22-0.48), with<br />
72<br />
a molecular weights (512.861 and 199.526)<br />
×10 3 Dalton respectively. One of these two<br />
bands (band-1) was shown to be different from<br />
the control as shown in table (1). This profile<br />
was found in 33 patients (55 %) Table (2).The<br />
third protein shows two bands the RM values<br />
were ranged from (0.48-0.50), with a<br />
molecular weights (199.526 and 181.970) ×10 3<br />
Dalton respectively. One of these two bands<br />
(band-2) was shown to be different from the<br />
control as shown in table (1). This profile was<br />
found in 7 patients (11.66 %) Table (2).<br />
Table 1 : RM values and mole cular weight for each band in different urine prote in profile groups.<br />
Band<br />
No.<br />
Control<br />
RM<br />
Control<br />
MW×1 3<br />
Dalton<br />
G1-<br />
RM<br />
G1-<br />
MW×10 3<br />
Dalton<br />
G2-<br />
RM<br />
G2-<br />
MW×10 3<br />
Dalton<br />
G3-<br />
RM<br />
G3-<br />
MW×1 3<br />
Dalton<br />
1 0.48 199.526 0.22 512.861 0.22 512.861 0.48 199.526<br />
2 0.32 316.227 0.48 199.526 0.50 181.970<br />
3 0.37 236.026<br />
4 0.48 199.526<br />
5 0.52 158.489<br />
RM: relative mobility, MW: molecular weight in Daltons.<br />
Table 2 : Diffe re nt urine protein profiles with the pe rcentage of patie nts in each group.<br />
Group Total No. bands No. abnormal bands No. Patie nts % patient<br />
1 5 4 20 33.4<br />
2 2 1 33 55<br />
3 2 1 7 11.6<br />
Discussion<br />
The detection and identification of trace<br />
amounts of proteins in complex samples is a<br />
major challenge in biomarkers discovery and<br />
validation (13, 14) . Samples of interest for<br />
detection of protein biomarkers are typically<br />
serum or plasma. However, with the rapidly<br />
growing interest in human urine proteome (15,<br />
16) , because of simplicity and<br />
noninvasiveness of collection, making urine an<br />
alternative sample type for many diagnostic<br />
tests (17) .Furthermore, urine contains a<br />
relatively small number of proteins typically<br />
present at low concentrations and thus simpler<br />
matrix for detecting proteins as compared to<br />
serum (17) . Some human diseases, excess<br />
proteins are found in the urine as can occur in<br />
patients with compromised kidney function.<br />
As a result, many of the proteins normally<br />
present in blood will also be excreted in to the<br />
urine. This condition is known as proteinuria,<br />
is often observed in acute inflammation, acute<br />
urinary tract infection, amyloidosis, diabetic<br />
nephropathy, kidney failure, multiple<br />
myeloma, nephrotic syndrome and severe<br />
yeast infection (18, 19 ) . Kidney involvement<br />
has been demonstrated in patients with Kalaazar<br />
with significant proteinuria (20) .The<br />
detection of Leishmania donovani soluble<br />
antigen and antibody (IgM and IgG) has also<br />
been shown in urine samples of kala-azar<br />
patients (21, 22).Abnormal renal functions<br />
particularly, glomerular filtration rate, urinary<br />
concentration, acidification that may be<br />
correlated to an immunological mechanisms<br />
and the evidence of renal proximal tubular<br />
damage have been demonstrated recently (23,<br />
24) . Lima et al. (2009) (24) have also detected<br />
the presence of different kinds of proteins in<br />
the urine of patients with Kala-azar<br />
particularly, albumin, IgG, β2-microglobulin,<br />
α1 , α2 , β and γ-globulins. All these studies<br />
had confirmed renal involvement during active<br />
Kala-azar disease. However, to the best of our<br />
knowledge, no study till now tried to<br />
demonstrate the types of protein patterns or<br />
profiles that may be observed in patients with<br />
the active disease. Therefore, in this study we<br />
tried to investigate-using urine samples- the<br />
presence of different protein profiles in a<br />
number of defined children with Kala-azar in
Iraqi J Pharm Sci, Vol.19(2) 2010 Protein profiles in children with Kala-azar<br />
Iraq using the conventional SDS-PAGE.The<br />
results of this study revealed the presence of<br />
one protein band in the control healthy<br />
children and the presence of three different<br />
protein patterns groups in the defined children<br />
with Kala-azar. As shown in the results,<br />
patients involved in this study were divided<br />
into three groups according to their urine<br />
protein profile (see the above results).Groups 2<br />
and 3 showed only 2 banding proteins in 55<br />
and 11.6 % of the studied patients, while group<br />
1 showed 5 different banding proteins in 33%<br />
of the studied patients. These differences in the<br />
number of bands for each banding pattern<br />
group may be attributable to the chronicity of<br />
infection (24) and to the difference in the<br />
degree of renal tubular dysfunction with<br />
differences in the types and amounts of<br />
proteins excreted in the urine (23, 24)<br />
.Furthermore, clinical and epidemiological<br />
studies in Iraq showed a large differences in<br />
the clinical picture of the disease in terms of<br />
fever, hepatomegally, spleenomegally (2, 25,<br />
26), anaemia, thrombocytopaenia and response<br />
to therapy (sodium stibogluconate) (26) .<br />
Similar findings have been reported in other<br />
endemic areas with Kala-azar like Sudan (27),<br />
India (28) and Iran (29). This may explain the<br />
different protein patterns among different<br />
groups in this study.An interesting finding was<br />
the presence of common band between group 1<br />
and 2 with a molecular weight (512.861×103)<br />
Dalton.Further investigations with expanded<br />
number of patients as well as identifying the<br />
types of protein for each banding pattern group<br />
especially for the common band which could<br />
be of diagnostic value.<br />
References<br />
1. Kharazmi, A., H. R. Rezai, M. Fani, and<br />
N. C. Behforouz. Evidence for the<br />
presence of circulating immune complexes<br />
in serum and C3b and c3d on red cells of<br />
Kala-azar patients. Trans Roy. Soc. Trop.<br />
Med. Hyg. 1982;76:793-6.<br />
2. Atia, L. A.. Hepatic involvement in<br />
visceral leishmaniasis and its relation to<br />
the severity of infection. Msc. Theses<br />
College of science Baghdaduniversity.1995.<br />
3. Jensen, A. T. R., A. Gaafar, A. Ismail, C.<br />
B. V. Christensen, M. Ke mp, A. M. El-<br />
Hassan, and T. Theauder. Serodiagnosis of<br />
leishmania donovani infections. Trans<br />
Roy. Soc. Trop. Med. Hyg. 1999;93:157-<br />
60.<br />
4. Cerf, B. J., T. C. Jones, R. Badaro, D.<br />
Sampaio, R. Teixeira, and, W. D. Jr<br />
Johnson. Malnutrition as a risk factor for<br />
73<br />
severe visceral leishmaniasis. J. Inf. Dis.<br />
1987;156:1030-33.<br />
5. Al-Bashir, N. M. T. Plasma protein<br />
profiles in Iraqi children with Kala-azar.<br />
Indian Journal of Clinical<br />
Biochemistry.(in press).<br />
6. Baqir, H. I., T. I. Al-Jeboori, and N. T. Al-<br />
Bashir. Detection of parasite antigen in the<br />
urine of patients with active visceral<br />
leishmaniasis. Diagnostic potential.<br />
2002;2(1):22-5.<br />
7. Islam, M.Z., M. Itoh, , S. M<br />
Shmsuzzaman, R. Mirza, F. Matin, I.<br />
Ahmed, A. K. M. S. Choudry, M. A.<br />
Hossain, X. G. Qiu, N. Begam, M.<br />
Furuya, J. L. Leafasia, Y. Hashinguchi,<br />
and, E. Kimura. Diagnosis of visceral<br />
leishmaniasis by ELISA using urine<br />
samples. Clinical diagnostic laboratory<br />
immunology. 2002;9:789-94.<br />
8. Al-Bashir, N. M. T., and S. H. Ali. The<br />
use of urine as a sample for the diagnosis<br />
of visceral leishmaniasis. The Iraqi journal<br />
of medical sciences. 2003;2(3):332-39.<br />
9. Islam, M. Z., M. Itoh, R. Mirza, I. Ahmed,<br />
A. R. M. S. Ekram, , A. H. Sarder, S. M.<br />
Shamsuzzaman, Y. Hashiguchi, and E.<br />
Kimura. Direct agglutination test with<br />
urine samples for the diagnosis of visceral<br />
leishmaniasis. Am J Trop Med Hyg.<br />
2004;70: 78–82.<br />
10. Lowery, Q. L., N. S. Rosenbrough,, A. L.<br />
Farr, and R. J. Randall. Protien<br />
mesurement with Folin-Phenol reagent.<br />
Journal of Biochemistry.1951;193:265-75.<br />
11. Fehrnstrom, H., and U. Morberg. SDS<br />
and conventional polyacrylamide gel<br />
electrophoresis with LKB2117 multiphor.<br />
LKB application note 306. Bromam,<br />
Sweden1977.<br />
12. Johnson, A., E. Rohfs, and M. Siverman.<br />
Protein. In. Tietz textbook of clinical<br />
chemistry. Eds. Burtis, C. A. & Ashwood.<br />
3rd ed. R. E. Saunders W. B. CO. London.<br />
1999;pp.477-530.<br />
13. Echan, L. A., H. Y. Tang, N. Ali-Khan, K.<br />
Lee, and D.W. Speicher. Depletion of<br />
multiple high-dependence protein<br />
improves protein profiling capacities of<br />
human serum and plasma. Proteomics.<br />
2005;5:3292-303.<br />
14. Sigdel, T. K., K. Lau, J. Schilling, and M.<br />
Sarwal. Optimizing protein recovery for<br />
urinary proteomics, a tool to monitor renal<br />
trasplantation. Clinical transplant.<br />
2008;22(5):617-23.<br />
15. Soldi, M., C. Sarto, and C. Valsecchi.<br />
Proteome profile of human urine with<br />
two-dimentional liquid phase<br />
fractionation. Proteomics.2005;5:2641-47.
Iraqi J Pharm Sci, Vol.19(2) 2010 Protein profiles in children with Kala-azar<br />
16. Thongboonkerd, V., T. Semangoen, and S.<br />
Chutipongtanate. Enrichment of<br />
basic/cationic urinary proteome using ion<br />
exchange chromatography and bath<br />
adsorption. Journal of Proteome<br />
Research.2007;6:1209-14.<br />
17. Kushnir, M. M., P. Morzinski, A. L.<br />
Rockwood, and D. K. Crockett. Depletion<br />
strategy for improved detection of human<br />
proteins from urine. Journal of<br />
Biochemical Techniques.2009;20: 101-08.<br />
18. Biesenbach G., K. Hommer, G. Stadler,<br />
R. Kramar, G. Syré, and J. Zazgornik.<br />
["Diabetic" proliferative retinopathy and<br />
nodular glomerulosclerosis without<br />
diabetes mellitus]. Dtsch Med<br />
Wochenschr. 1988;16,113(50):1968-71.<br />
19. van Ypersele de Strihou, C., and Y.<br />
Pirson. Indications for dialysis and<br />
trasplantation in end-stage renal diseae.<br />
Contrib Nephrology. 1989;71:1104-10.<br />
20. Weisinger, José R., A. Pinto, G. A.<br />
Velazquez, I. Bronstein, J. J. Dessene, J.<br />
F. Duque, J. Montenegro, F. Tapanes, and<br />
A. R. de Rousse. Clinical and Histological<br />
Kidney Involvement in Human Kala-Azar.<br />
Am. J.Trop. Med. Hyg.1978 ;27(2) : 357-<br />
359.<br />
21. Kohanteb, J., S. M. Ardehali, and H. R.<br />
Rezai. Detection of Leishmania donovani<br />
soluble antigen and antibody in the urine<br />
of visceral leishmaniasis patients. Trans R<br />
Soc Trop Med Hyg. 1987; 81(4):578-80.<br />
22. Mebrahtu, Y. B., L. D. Hendricks, C. N.<br />
Oster, P. G. Lawyer, P. V. Perkins, H.<br />
Pamba, D. Koech, and C. R. Roberts.<br />
Leishmania donovani parasites in the<br />
nasal secretions, tonsillopharyngeal<br />
mucosa, and urine centrifugates of visceral<br />
leishmaniasis patients in Kenya. Am J<br />
Trop Med Hyg. 1993;48(4):530-5.<br />
74<br />
23. Lima Verde, F. A., F. A. Lima Verde, I.<br />
A. Lima Verde, G. B. Silva Junior, E. F.<br />
Daher, and E. M. Lima Verde. Evaluation<br />
of renal function in human visceral<br />
leishmaniasis (kala-azar): a prospective<br />
study on 50 patients from Brazil. J<br />
Nephrol. 2007;20(4):430-6.<br />
24. Lima Verde, F. A., F. A. Lima Verde, E.<br />
F. Daher, G. M. Dos Santo, and E. M.<br />
Lima Verde. Renal tubular dysfunction in<br />
human visceral leihmaniasis (Kala-azar).<br />
Clinical Nephrology, 2009;71(5):492-500.<br />
25. Abas, M. Z., and A. M. Jabri. Clinicoepidemiological<br />
study of kala-azar in Iraq.<br />
A thesis submitted to the scientific council<br />
of Paediatrics. Baghdad.1998.<br />
26. Ali, S. H., N. T. Al-Bashir, and D. M.<br />
Thamiri. Clinical and epidemiological<br />
study of visceral leishmaniasis in a group<br />
of hospitaized children in Baghdad. Iraqi<br />
paediatric Medicine Journal, 2003;2(4):<br />
371-77.<br />
27. Zijlstra, E. E., M .S. Ali, A. M. el-<br />
Hassan, I. A. el-Toum,, M. Satti, H.W.<br />
Ghalib, E. Sondorp, and A. Winkler.<br />
Kala-azar in displaced people from<br />
southern Sudan: epidemiological, clinical<br />
and therapeutic findings. Trans R Soc<br />
Trop Med Hyg. 1991;85(3):365-9.<br />
28. Mittal, V., R. Bahatia, and S. Sehgal.<br />
Clinocoepidemiological profile of Kalaazar<br />
patients in Delhi. Journal of<br />
Communicable Diseases, 1989;21(4) :<br />
255-61.<br />
29. Soleimanzadech, G., G. H. Edrissian, A.<br />
M. Movahhed, Danesh, and A. Nadim.<br />
Epidemiological aspect of Kala-azar in<br />
Meshkin-Shahr, Iran (human infection).<br />
Bull WHO. 1993;71(6): 759-62.
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
Development of Modified Release Nicotine Tablet Formulation for<br />
Treatment of Ulcerative Colitis<br />
Marwan Y. Al-hurr *,1<br />
* Department of Pharmaceutics, College of Pharmacy, University of Baghdad,Baghdad,Iraq.<br />
Abstract<br />
One of the therapeutic effects of nicotine is used as a protective against developing ulcerative<br />
colitis . ulcerative colitis is an inflammatory disease of the bowel affecting the superficial lining<br />
mucosa in the rectum and large intestine. In this study nicotine tablets were formulated as modified<br />
release tablets targeted to the colon. All formulas were studied for drug release , effect of diluent,<br />
retardant concentration, avicel grade,and compression force, the selected formula was then further<br />
studied for drug release in 3 different pH ( coated tablets) .The kinetic study revealed acceptable shelf<br />
life . Finally the selected formula was given to 6 patients in a pre-liminary clinical study which showed<br />
that nicotine can stabilize mild to moderate ulcerative colitis attacks.<br />
Key words: Ulcerative colitis, Nicotine, Modifie d release , Colon delivery.<br />
ﺔﺻﻼﺨﻟﺍ<br />
ﻪﻈﻴﻠﻐﻟﺍ ءﺎﻌﻣﻻﺍ ﻭ ﻢﻴﻘﺘﺴﻤﻠﻟ ﺔﻴﺤﻄﺴﻟﺍ ﺔﻴﻃﺎﺨﻤﻟﺍ ﺔﻘﺒﻄﻟﺍ ﺐﻴﺼﺗ ﻲﺘﻟﺍ ﻲﻤﻀﻬﻟﺍ ﺯﺎﻬﺠﻟﺍ ﺕﺎﺑﺎﻬﺘﻟﺍ ﺪﺣﺍ ﻲﺣﺮﻘﺘﻟﺍ ﻥﻮﻟﻮﻘﻟﺍ ﺏﺎﻬﺘﻟﺍ<br />
ﻰﻟﺍ ﻪﺟﻮﻣ ﺭﺮﺤﺘﻟﺍ ﺓﺭﻮﺤﻣ ﻦﻴﺗﻮﻜﻴﻨﻟﺍ ﺕﺎﻃﻮﻐﻀﻣ ﺮﻴﻀﺤﺗ ﻢﺗ ﺚﺤﺒﻟﺍ ﺍﺬﻫ ﻲﻓ . ﺽﺮﻤﻟﺍ ﺍﺬﻫ ﺭﻮﻄﺗ ﺪﺿ ﺔﻳﺎﻤﺣ ﻞﻣﺎﻋ ﻦﻴﺗﻮﻜﻴﻨﻟﺍ ﺮﺒﺘﻌﻳﻭ<br />
ﺓﻮﻗ ﺮﻴﺛﺄﺗﻭ , ﺓﺮﻀﺤﻤﻟﺍ ﻎﻴﺼﻠﻟ ﺭﺮﺤﺘﻠﻟ ﻪﻄﺒﺜﻤﻟﺍ ﺩﺍﻮﻤﻟﺍ ﺰﻴﻛﺮﺗ ﻭ ﺔﻟﺎﻌﻔﻟﺍ ﺮﻴﻏ ﺔﻓﺎﻀﻤﻟﺍ ﺩﺍﻮﻤﻟﺍ ﻉﻮﻧ ﺮﻴﺛﺄﺗ ﻭ ءﺍﻭﺪﻟﺍﺭﺮﺤﺗ ﺔﺳﺍﺭﺩ ﻢﺗﻭ . ﻥﻮﻟﻮﻘﻟﺍ<br />
ﻭ ﻲﻨﻴﺟﻭﺭﺪﻴﻬﻟﺍ ﺱﻻﺍ ﺔﻔﻠﺘﺨﻣ ﻁﺎﺳﻭﺍ ﻲﻓ ﻦﻴﺗﻮﻜﻴﻨﻟﺍ ﺭﺮﺤﺗ<br />
ﺚﻴﺣ ﻦﻣ ﺓﺭﺎﺘﺨﻤﻟﺍ ﻪﻐﻴﺼﻠﻟ ﻊﺳﻭﺍ ﺔﺳﺍﺭﺩ ءﺍﺮﺟﺃ ﻢﺗﻭ . ﻦﻴﺗﻮﻜﻨﻟﺍ ﺭﺮﺤﺗ ﻰﻠﻋ ﺲﺒﻜﻟﺍ<br />
ﺕﺮﻬﻇﺃ ﺚﻴﺣ ﺓﺭﺎﺘﺨﻤﻟﺍ ﺔﻐﻴﺼﻠﻟ ﻰﺿﺮﻣ 6 ﻰﻠﻋ ﺔﻴﻟﻭﺍ ﻪﻳﺮﻳﺮﺳ ﺔﺳﺍﺭﺩ ءﺍﺮﺟﺍ ﺎﻀﻳﺍ ﻢﺗﻭ . ﺔﻔﻠﺘﺨﻣ ﺓﺭﺍﺮﺣ ﺕﺎﺟﺭﺩ ﻲﻓ ﺎﻬﺗﺎﺒﺛ ﺚﻴﺣ ﻦﻣ<br />
. ﺓﺪﺸﻟﺍ ﺔﻟﺪﺘﻌﻤﻟﺍ ﻭ ﺔﻄﻴﺴﺒﻟﺍ ﻞﺣﺍﺮﻟﺍ ﻲﻓ ﺽﺮﻤﻟﺍ ﺍﺬﻫ ﺔﻳﺭﺍﺮﻘﺘﺳﺍ ﻦﻣ ﺪﻳﺰﻳ ﻭ ﺽﺮﻤﻟﺍ ﺭﻮﻄﺗ ﻊﻨﻤﻳ ﻦﻴﺗﻮﻜﻴﻨﻟﺍ ﻥﺍ ﺔﺳﺍﺭﺪﻟﺍ<br />
Introduction<br />
Colonic drug delivery has gained<br />
increased importance not just for the delivery<br />
of the drugs for the treatment of local disease<br />
associated with the colon like Crohns disease ,<br />
ulcerative colitis and irritable bowel<br />
syndrome..etc., but also for the potential<br />
systemic delivery of proteins and therapeutic<br />
peptides. The large intestine, though difficult<br />
to reach by preoral delivery is still deemed to<br />
the delivery of agents to cure the local disease<br />
of the colon (1,2) . Colonic delivery formulations<br />
are in general may be designed to provide<br />
either the burst release or for sustained/<br />
prolonged release once reaching the colon (3) .<br />
The proper selection of a formulation approach<br />
depends upon several important factors (4)<br />
which are : the pathology and pattern of the<br />
disease , the physicochemical and<br />
biopharmatical properties of the drug and<br />
finally the desired release profile of the active<br />
ingredient. The most common physiological<br />
factors considered in the design of delayed<br />
release colonic formulations is pH gradient of<br />
the gastrointestinal tract (5,6) .delayed release<br />
formulations such as single unit or<br />
multiparticulate system for colon targeting ,<br />
nanoparticulate system, microspheres,<br />
pelletsand beads, coating with pH sensitive<br />
polymers, embedding in matrices and<br />
bioadhesive systems (7) can be considered for<br />
75<br />
the design of colon deliveryformulations. A<br />
wide array of polymers has been employed as<br />
drug release retarding agents each of which<br />
presents a different approach to matrix<br />
concept. Plastic matrix system , due to their<br />
chemical inertness and drug embedding ability<br />
, have been widely used for sustaining the<br />
release of drugs. Plastic polymers e.g.<br />
ethylcellulose and acrylates, which are capable<br />
of forming insoluble or skeleton matrices, have<br />
been widely used for controlled release of<br />
drugs due to their inertness and drug<br />
embedding ability . Acrylate polymers are<br />
widely used as tabletcoating and as retardents<br />
for drug release in sustained release<br />
formulations (8) . Ulcerative colitis (U.C) is an<br />
inflamma tory disease of the bowel affecting<br />
the superficial lining mucosa in rectum and<br />
large intestine. The disease typically starts<br />
from the rectum and continues through the<br />
large bowel sparing the deeper layers of the<br />
intestinal wall<br />
(9) . A variety of anti-<br />
inflamma tory, immunosuppressive, and<br />
biological agents have been used to induce and<br />
/ or maintain remission in UC . Sulfasalazin,<br />
olsalazine, balsalazide, oral and rectal<br />
mesalamine and topical corticosteroids are the<br />
standard first line therapies for UC.Patients<br />
who fail to respond to these agents are usually<br />
treated with oral corticosteroids.<br />
1Corresponding author E- mail : m@marwanpharm.com , mngateway2000@yahoo.com<br />
Received : 1/ 9/ 2010<br />
Accepted : 11/12/2010
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
There is a need for additional first line<br />
treatments in patients with UC and for<br />
alternatives to corticosteroid therapy in<br />
refractory patients. Nicotine may be such an<br />
agent for which epidemiologic studies have<br />
shown that smoking protects against the<br />
development of UC and controlled clinical<br />
trials have demonstrated that transdermal<br />
nicotine is efficacious for active UC (10-15) .<br />
Nicotine is a drug obtained from the plant<br />
Nicotina tabacum. It’s a weak base. Its<br />
available as a colorless to pale yellow oily<br />
liquid with an unpleasant tobacco-like odour<br />
and burning taste (16) . Its most preferred that<br />
absorption of nicotine occurs predominantly in<br />
the colon. So post-gastric delayed release<br />
composition, the composition will pass through<br />
the small intestine in about 4-8hr and will<br />
resides in the colon for about 48hr. further<br />
more nicotine is absorbed more slowly in the<br />
colon than in the small intestine, therefore,<br />
nicotine delivered for absorption<br />
predominantly in the colon will be absorbed<br />
more slowly over a sustained period and will<br />
give rise to more uniform plasma<br />
concentration. By predominant absorption<br />
from the colon we mean to include preferably<br />
80-90% of the total dose of nicotine (17) .The<br />
present study is to develop modified release of<br />
20mg nicotine tablets as a targeted delivery<br />
system in the colon with pre-liminary clinical<br />
study. Eudragit RS PM as a retardant polymer<br />
which is responsible for the sustained release<br />
behavior of the drug was used for preparation<br />
of the core matrix and the selected formula was<br />
76<br />
targeted to release in the colon by using enteric<br />
coating ( a mixture of Eudragit L and S ).<br />
Materials and Method<br />
Nicotine (Sigma) gifted from<br />
Pharmacognosy Department. College of<br />
Pharmacy/ University of Baghdad, Sodium<br />
hydroxide (Fluka chemie AG. Buchs/Scheiz),<br />
Dibutylphthalate ( USB, B. Brussels,Belgium),<br />
Hydrochloric acid , Iospropanol, and<br />
Orthophosphoric acid (Riedal De Haen Ag<br />
Seelze Hanover), Polyvinylpyrrolidone ( PVP<br />
K30 ) , Acetone, Potassium dihydrogen<br />
phosphate, Ethanol 99% ( BDH chemicals,<br />
Ltd, Liverpool, England )Microcrystalline<br />
cellulose- Avicel ® - PH101, PH302, PH200<br />
(FMC Corperation, Pennsylvania, USA),<br />
Eudragit ® L100, S100, RS PM – Rhö m<br />
Pharma GmbH Weiterstadt,<br />
Germany),Trisodium phosphate, Talc<br />
(Hopkins and Williams Ltd. England), coloring<br />
agent ( deep orange lakes) Zinc stearate<br />
(Barlocher, GmbH, Germany), Disodium<br />
hydrogen phosphate, Mannitol, Starch ( Merk,<br />
Germany). Table (1) summarizes 8 formulas to<br />
prepare modified release nicotine tablets by<br />
wet granulation method with alcohol. A known<br />
weight of the granules were mixed with a<br />
speicified amount of Zn stearate ( 1%) in a<br />
well closed container and compressed into<br />
tablets using 9mm punches ( tablet machine<br />
single punch – Korch, type EKO, Erweka<br />
GmbH, Kr Offenbanch/ Germany ).<br />
Table 1 : Different formulas of nicotine pre pared as modifie d re le ase tablets.<br />
Constituents<br />
1 2 3<br />
Formulas ( mg)<br />
4 5 6 7 8<br />
Nicotine 20 20 20 20 20 20 20 20<br />
PVP (10%) 20 20 20 20 20 20 20 20<br />
Avicel ® PH302 40 40 40 40 40 40<br />
Eudragit ® RS PM 20 20 40 60 20 20 20 20<br />
Starch 100 100 100 100 100 100 100<br />
Zinc stearate 1% 1% 1% 1% 1% 1% 1% 1%<br />
Mannitol 100<br />
Avicel ® PH101 40<br />
Avicel ® PH200 40<br />
Compression force 4Kg 4Kg 4Kg 4Kg 4Kg 4Kg 6Kg 8Kg<br />
Total weight of final tablet 200 200 200 200 200 200 200 200<br />
Evaluation of the prepared tablets<br />
The following parameters were used to<br />
compare the prepared formulas to obtain the<br />
final selected formula.<br />
1. Effect of dilue nts type on the pe rcent<br />
re leased of nicotine .<br />
Formula 1 and 2 were used to study the<br />
effect of two different diluents ( starch<br />
andmannitol ) on drug release.<br />
2. Effect of Eudragit RS PM conce ntration.<br />
Formula 1,3 and 4 were utilized to study<br />
the effect of different concentrations (10%<br />
, 20% and 30% respectively) of Eudragit<br />
RS PM on the drug release.
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
3. Effect of Avicel grade.<br />
Formulas 1,5 and6 which contain Avecil<br />
PH 302, PH101,and PH200 respectively<br />
were used to study the effect of different<br />
grades of Avecil as channeling agent on the<br />
drug release.<br />
4. Effect of compre ssion force on nicotine<br />
re lease.<br />
Formulas 1, 7and 8 were used to study the<br />
effect of changing the compression force<br />
4Kg, 6Kg and 8Kg respectively on the drug<br />
release.<br />
Drug release : ( USP dissolutuion apparatus<br />
type II, Coply scientific Ltd, England)<br />
The Medium used : pH 6.8 phosphate<br />
buffer 750ml, Apparatus II , rotation 75 rpm,<br />
with a Sampling time: 1,2,3,4 and 6hr. The<br />
amount of nicotine dissolved was determined<br />
spectrophotometecally at λmax. 260nm of<br />
filtered samples.( UV visible<br />
spectrophotometer, Carrywin UV. Varian,<br />
Australia). The samples were diluted with<br />
dissolution medium if necessary and compare<br />
with a standard solution having a known<br />
concentration of nicotine in the same medium.<br />
Assay: HPLC analytical method<br />
The chromatographic separation was<br />
achieved on a C-18 colum with UV detection<br />
at 260nm the HPLC system comprised a<br />
(Waters 1500 series HPLC pump( USA) ,<br />
waters 2487 dual λ absorbance detecter, water<br />
breeze soft ware.) was operated at ambient<br />
temperature and used citrate buffer: methanol<br />
(85: 15 % v/v ) with an appearent pH 2.4 as the<br />
mobile phase. The flow rate was maintained at<br />
0.7 ml / min. and the retention time 6.94<br />
min. (18) .<br />
Preparation of coating formula<br />
The coating solution was prepared<br />
according to the Rhom pharma<br />
recommendations (the manufacturer) as<br />
follows:<br />
Formula :<br />
Eudragit * 6gm<br />
isoprpanol 115.7gm<br />
acetone 77.1gm<br />
Dibutylphthalate 1.2gm<br />
talc 3.25gm<br />
Magnesium stearate 0.25gm<br />
Color 0.25gm<br />
Titanium oxide 1.55gm<br />
Semithicone Q.S.<br />
*mixture of Eudragit L 100 and S 100 in a<br />
ratio 1:2<br />
77<br />
The final coating solution formula prepared<br />
was 205.3gm.<br />
Procedure<br />
The formula was prepared by mixing the<br />
solvents together with the plasticizer (<br />
dibutylphthalate) in a high shear mixer MLW<br />
type LR10 ( VEB ML W Prufgerate-werk,<br />
Medingen/ Stizfrtal/ Germany). Eudragit<br />
mixture was added slowly at room temperature<br />
, the powder was thoroughly wetted and care<br />
was taken to ensure that nothing settled at the<br />
bottom or formed lumps . mixing lasted for at<br />
least 30mins, until the solution was clear, the<br />
fillers were added step by step .<br />
Calculation<br />
( 19)<br />
required<br />
of the amount of lacquer<br />
A specific thickness of coating is<br />
required based on the purpose of the coating<br />
and the amount needed depending on the<br />
surface area of the cores which may be<br />
calculated from the following equation<br />
assuming tha t the tablets are cylindrical in<br />
shape:<br />
S.A= Л ( d.h + ½ d 2 )<br />
Where d is the diameter (mm)<br />
h is the height (mm)<br />
S.A is the surface area (mm 2 )<br />
The nicotine tablets had a diameter of 9mm<br />
and a surface area approximately equals to<br />
240mm 2 .<br />
Since 3-5mg lacquer / cm 2 of tablet cores<br />
required to produce a core resistant to acidic<br />
environment ( enteric coated tablets). So<br />
multiplying the surface area of the tablet core<br />
by the amount required and dividing it by the<br />
weight of tablet, the quantity o f the lacquer to<br />
be applied as a percentage will be obtained.<br />
The amount to be applied<br />
( % dry lacquer substace)= S.A( mm 2 ) / w (<br />
mg) x ( mg/ mm 2 )<br />
= 240 / 200 x 5<br />
= 6%<br />
Tablet coating<br />
The selected Formula was coated by<br />
dipping method . each tablet was held by<br />
forcipes and dipped in the coating lacquer in<br />
and out 15-20 times , the coat was dried by a<br />
.( 20)<br />
stream of warm air between each dip<br />
( 21)<br />
Dissolution study of the coated tablets<br />
The dissolution rate of the selected<br />
formula for nicotine ( coated tablets) was<br />
determined using USP apparatus at 37+ 0.5 o C<br />
with paddle and the rotation speed was set at<br />
75rpm in order to simulate the pH change of
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
the GIT , pH change dissolution procedure was<br />
applied as follows:<br />
2hr. testing in 0.1N HCl solution followed by<br />
testing for one hour in phosphate buffer pH 4<br />
obtained by adding 195ml 0.2M tribasic<br />
sodium phosphate solution during which<br />
samples were withdrawn at specified times and<br />
replaced immediately by fresh medium. Then<br />
the medium was changed to pH 6.8 by adding<br />
55ml 0.2 M tribasic sodium phosphate adjusted<br />
by 2N NaOH or 2N HCl if required . samples<br />
were withdrawn at different time intervals and<br />
analyzed spectrophotometrically at 260nm.<br />
Kinetic study<br />
Effect of temperature:<br />
The effect of temperature on the<br />
degradation of the selected formula of nicotine<br />
modified release tablets was studied . The<br />
study was done by storing 90 tablets in ovens<br />
(Mermert UL 80 ( Rostfrei, Schwach,<br />
Germany) )at different temperatures 50 o C,<br />
60 o C and 70 o C.<br />
Samples were taken at specified time intervals<br />
and analyzed for nicotine. Since the<br />
degradation of the drug follows 1 st order<br />
kinetics, the expiration date t10% at 25 o C could<br />
be calculated using the following equation :<br />
T10% = 0.105/ k 25 o C<br />
Pre-liminary clinical study<br />
Before giving the preparation we<br />
obtained a written consent of the patients who<br />
were included in this study. The modified<br />
release nicotine tablets of the selected formula<br />
was given to 6 patients suffering from mild to<br />
moderate ulcerative colitis (high mucus<br />
secretions, irritable bowl syndrome, mild to<br />
moderate bleeding and gases). All patients<br />
were put on 20mg single dose of nicotine for 2<br />
weeks. The patients were evaluated<br />
clinically(physical examination and endoscopy<br />
) before and during treatment ( physical<br />
examination ) under the supervion of Dr.<br />
Mumtaz k. Hanna at his private clinic.<br />
Results and discussion<br />
Effect of diluent’s type on nicotine release<br />
Although diluents are normally thought<br />
to be inert ingredients they can significantly<br />
affect the biopharmaceutical, chemical and<br />
physical properties of the final tablets .(22)<br />
Formula 1and 2 which contain maize starch<br />
and mannitol as diluents, it was seen that<br />
starch gave the best drug release compared<br />
with mannitolas shown in fig. 1 .This behavior<br />
may be attributed to the swellability property<br />
of starch when compared with mannitol which<br />
the release is due to water solubility. (23)<br />
78<br />
% Drug Released<br />
Figure 1: Effect of dilue nt type on the<br />
release of nicotine at pH 6.8 and 37 o C<br />
Effect of Eudragit concentration:<br />
Eudragit RS PM can be in corperated in<br />
a percent of 10-30% (w/w) by weight to<br />
provide suitable granules and matrix tablets.<br />
The amout of Eudragit RS PM to be added ,<br />
depends upon the solubility characteristics of<br />
the drugs and the rate required (20). Formulas 1 ,<br />
3 and 4 which contain 10% , 20% and 30 %<br />
w/w of Eudragit ( as a retardant) respectively<br />
were evaluated . formula 1 gave the best<br />
modified release of nicotine when compared<br />
with the requirements of drug release to the<br />
colon (17). The results from dissolution profiles<br />
of formulas 1 ,3 and 4 indicates that the<br />
retardant content affects the release of nicotine<br />
from the tablet, this result is in a consistent<br />
with the results obtained when Eudragit RSPM<br />
polymer was used as a retardant material for<br />
diclofenac sodium and indomethacin tablets. (24)<br />
It appears that the amount of retardant needed<br />
is 10 % as shown in fig. 2 .This is in<br />
agreement with the reported data which<br />
indicated that the retardation effect on the<br />
release of drug is dependent on the amount of<br />
Eudragit included (25)<br />
%Drug Released<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
0 1 2 3 4 6<br />
Time ( hr.)<br />
0 1 2 3 4 6<br />
Time ( hr.)<br />
10% eudragit<br />
RSPM<br />
20% eudragit<br />
SRPM<br />
30% eudragit<br />
RSPM<br />
starch<br />
Figure 2 : Effect of Eudragit RS PM<br />
concentration on the percent re leased of<br />
nicotine at pH 6.8 and 37 o C.<br />
mannitol
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
Effect of Avicel grade<br />
Avicel is microcrystalline cellulose , it is<br />
partially depolymerized cellulose prepared by<br />
treating alpha- cellulose obtained as a pulp<br />
fibrous plant material with acids. The grade of<br />
Avicel depends on its normal loss on drying ,<br />
bulk density and degree of polymerization<br />
values. (26 ) The results showed that formula 1<br />
in which Avicel PH 302 present, gave the best<br />
dissolution profiles while the difference in the<br />
release that occurred in the other formulas 5<br />
and 6 which contain Avicel PH 101 and PH<br />
200, respectively. is due to the difference in the<br />
porosity , surface area, particle size and density<br />
of Avicel as stated (27 ) as shown in fig. 3<br />
%Drug Released<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
0 1 2 3 4 6<br />
Time ( hr.)<br />
Figure 3 : Effect of Avicel grade on the perce nt<br />
release d of nicotine at pH6.8 and 37 o C.<br />
Effect of compression force<br />
Compression forces affect the hardness<br />
of a tablet and its thickness at a constant die fill<br />
as additional compression force is applied , the<br />
hardness values increase and the thickness<br />
decrease and the decrease as shown in fig.4<br />
( 19)<br />
, thereby the dissolution of the drug decrease in<br />
surface area and increase in hardness. This<br />
may be related to the low porosity resulted<br />
from the increase in compression force. (8)<br />
Therefore, formula 1 was selected because it<br />
gave the best drug release profile which<br />
complies with absorption properties of nicotine<br />
from the colon and it was further investigated<br />
for enteric coating, kinetic study and preliminary<br />
clinical study (17).<br />
Figure 4 : Effect of compression force on<br />
perce nt release d of nicotine at pH 6.8 and<br />
37 0 C.<br />
Avicel<br />
PH302<br />
Avicel PH<br />
101<br />
Avicel PH<br />
200<br />
79<br />
Coating formula<br />
The tablets showed good appearance with<br />
no signs of craking or splitting or peeling.<br />
Induction of hydrophobic materials and inert<br />
fillers ( Mg stearate , talc , titanium oxide ,<br />
aluma lakes with an orange color ) these fillers<br />
facilitate processing of the lacquer by reducing<br />
its stickness, help to smooth the permeability<br />
to water and decrease the tackiness of the<br />
drying lacquer. In addition they reduce the<br />
permeability of the film as long as the<br />
mechanical strength is maintained thereby<br />
enhancing the enteric properties of the film (<br />
28,29 ). Formula 1 was coated to target the drug<br />
to the colon. The coated tablets showed no<br />
drug release in 0.1 N HCl for 2hr. period of<br />
the test and the release of the drug increased<br />
rapidly when the pH changed to 6.8 as shown<br />
in fig 5.<br />
% Drug Released<br />
100<br />
pH1.2<br />
80<br />
pH 4 pH 6.8<br />
60<br />
40<br />
20<br />
0<br />
1 2 3 4 5 6 7 8 9 10<br />
Time (hr)<br />
Figure 5 : The cumulative percent of enteric<br />
coate d nicotine table ts release at diffe rent<br />
pH-media at 37 o C.<br />
Kinetic study<br />
Effect of temperature<br />
The stability of the coated modified<br />
release tablets were studied at different<br />
exaggerated temperatures ( 50 o C , 60 o C and<br />
70 o C ) for 3 months. Fig 6 shows the change in<br />
the log percentage remaining of nicotine versus<br />
time at different temperatures. The obtained<br />
profiles were linear , indicating that nicotine<br />
degradation follows 1 st order kinetics. The<br />
slopes of these lines were determined and the<br />
calculated rate constants (k) are summarized in<br />
fig (6). Arrhenius plot was then constructed as<br />
shown in fig 7. the linearty of the curve<br />
indicates its utility in predicting the rate of<br />
degradation at lower temperatures. since the<br />
degradation of the drug followed 1 st order<br />
kinetics the expiration date can be calculated at<br />
25 o C for nicotine coated matrix tablets and it<br />
was 52 month.
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
ln percent remaining<br />
Figure 6 : Perce nt remaining of drug versus<br />
time.<br />
log K<br />
4.65<br />
4.6<br />
4.55<br />
4.5<br />
4.45<br />
4.4<br />
4.35<br />
0<br />
-0.5<br />
-1<br />
-1.5<br />
-2<br />
-2.5<br />
-3<br />
0 1 2 3<br />
Time( month)<br />
Fig7: Arrhinous plot for expiration date<br />
estimation of nicotine tablet (Formula 1).<br />
50 C<br />
60 C<br />
70 C<br />
0.0028 0.0029 0.003 0.0031 0.0032 0.0033 0.0034<br />
Clinical study<br />
Nicotine was given to 6 non smoking<br />
patients ( 5 males and 1 female ) with an age<br />
range of 27- 65 yr. The patients took 20mg<br />
once a day for 10 days suffering from mild to<br />
moderate ulcerative colitis. The out come of<br />
this preliminary study indicates that 67% ( 4<br />
out of 6 patient ) were responsive to nicotine<br />
therapy ( relief of bleeding and most sign and<br />
sympto ms) although all patients suffered from<br />
adverse effect reaction towards nicotine<br />
therapy because the patient were non smokers<br />
(lightheadedness or dizziness, nausea,<br />
headaches, central nervous system stimulation<br />
and tachycardia). (30) further studies in the<br />
future should be done including in vivo<br />
nicotine blood concentration to optimize the<br />
dose.<br />
References<br />
1. Davis SS. Overcoming barriers to the oral<br />
administrationof peptide drugs. Trends<br />
Pharm Sci 1990;11:353-5.<br />
2. Vandenmooter G, Kinget R. Oral colonspecific<br />
drug delivery: A review. Drug<br />
Delivery 1995;2:81-93.<br />
3. Pinhasi A, Gomberg M, Avramoff A.<br />
US200467030442004.<br />
1/T<br />
4. Brahma NS. Modified – release solid<br />
formulations for colonic delivery. Recent<br />
pat drug deliv formul 2007;1:53-63.<br />
5. Evans DF, Pye G, Bramley R,Clark AG,<br />
Dyson TJ, Hardcastle JD. Measurement<br />
of gastrointestinal pH profiles in normal<br />
ambulant human subjects. Gut 1988;<br />
29:1035-41.<br />
6. Nugent SG, Kumar D, Rampton DS,<br />
Evans DF. Intestinal luminal pH in<br />
inflamma tory bowel disease: Possible<br />
determinants and implications for therapy<br />
with aminosalicylatesand other drugs.<br />
Gut 2001;48:571-7.<br />
7. Das S, Deshmukh R, Jha AK. Role of<br />
natural polymers in the development of<br />
multipartculate systems for colon drug<br />
targeting. Systematic Reviews in<br />
pharmacy 2010;1:1:70-85.<br />
8. Uddin MB, Chowdhury JA, Azam KR,<br />
Islam MK. Investigation of the effects of<br />
different physiochemical parameters on<br />
in vitro release kinetics of theophylline<br />
from Eudragit NE 30 and Eudraget RS<br />
30D matrix tablets. J. pharm. Sci. and Res<br />
2010; 2(4):240-46.<br />
9. Friedmans S, Blumberg R, Inflamma tory<br />
bowel disease In ; Fauci AS , Braunwald<br />
E, Kasper DL, Hauser SL, Longo DL,<br />
Jameson JL, et al , editors Harrison’s<br />
principles of internal medicine 17 th ed.<br />
Mc Graw Hill; 2008 p. 1777-89.<br />
10. Wolf JM, Lashner BA, Inflamma tory<br />
bowel disease: sorting out treatment<br />
options. Cleveland Clin. J. Med.<br />
2002;69:621-31.<br />
11. Kozuch PL, Hanauer SB. Treatment of<br />
inflamma tory bowel disease: a review in<br />
medical therapy. World J. Gastroenterol.<br />
2008;14:354-77.<br />
12. Lashner BA, Hanauer SB, Silverstien<br />
MD. Testing nicotine gum for ulcerative<br />
colitis patients: experience with single<br />
patient trials. Dig. Dis. Sci. 1990;35:827-<br />
32.<br />
13. Pullan RD, Rhodes J, Ganesh S, Manni<br />
V, Morris JS, Williams GI, et al.<br />
Transdermal nicotine for active ulcerative<br />
colitis . N Eng. J. Med. 1994; 330: 811-5.<br />
14. Guslandi M, Nicotine treatment of<br />
ulcerative colitis . Br. J. Pharmacol.<br />
1999;48:481-4.<br />
15. Boyko EJ, Perera DR, Koepsell TD, et al.<br />
Effect of cigarette smoking on the clinical<br />
course of ulcerative colitis. Scand. J.<br />
Gastroenterol. 1988;23:1147-52.<br />
16. Remington: The science and practice of<br />
pharmacy, 21 st ed. Lippincot,Williams<br />
and Wilkins, USA (2006), 1391
Iraqi J Pharm Sci, Vol.19(2) 2010 Modified release nicotine tablet<br />
17. Rhodes J., Evans B., Rhodes P., Sandorn<br />
W.; Intestinal absorption of nicotine<br />
responsive conditions; J. Mayo education<br />
and research: 1999; 6:238.<br />
18. Dash A.K., Wong S.T., Liquid<br />
chromatographic method for the<br />
determination of nicotine in<br />
pharmaceutical formulation. J.<br />
Chromatogr. A. 1996: 749: 81-85.<br />
19. Rohm pharma information sheets , Rohm<br />
Pharma GMbH, Weiterstadt; info.<br />
Eudragit L100-55 a/e , Eudragit S100 4/e<br />
, 1/s-7e 2006.<br />
20. Lachman L., Lieberman H.A., Kanig<br />
J.A; the theory and practice of industrial<br />
pharmacy 3 rd ed. Leo and febiger . chap. :<br />
1986;12:372.<br />
21. USP 2007 compact disk.<br />
22. Banker G., Baley P., baley G.; tablet<br />
formulation and design, liberman and<br />
Lachman (Eds) pharmaceutical dosage<br />
form tablets, vol.1 , Marcel Deckker,<br />
New York 1982; 61-108.<br />
23. Laako R., Eerkainen S.;Effect of core<br />
components on indomethacin release<br />
fro m film-coated granules; int.J. Pharm.<br />
:1991; 67(1):79-88.<br />
24. Al-hurr M.Y. ; formulation andclinical<br />
study of diclofenac sodium and<br />
81<br />
indomethacin as an oral modified release<br />
tablets, thesis for M.Sc. degree, College<br />
of Pharmacy, University of Baghdad,<br />
2003.<br />
25. Khanfar M.S., Salem M.S. Najib N.M.,<br />
Pillai G.K, Dissolution Behavior of<br />
sustained release formulation of<br />
indomethacin with Eudragit RS Acta.<br />
Pharm. Hung 1997; 67(6):235-239.<br />
26. Handbook of pharmaceutical excipients<br />
6 th ed. Washington DC., American<br />
pharmaceutical association 2009.<br />
27. Al-Ebady M.N. ; Design and in vitro<br />
evaluation of Prednisolone tablets as a<br />
potential colon delivery system, thesis for<br />
M.Sc. degree, college of pharmacy,<br />
university of Baghdad, 2006.<br />
28. Chatfield H.W,; Science of surface<br />
coating, Van Nostrand, NewYork 1962.<br />
29. Kassab H.J., Formulation and clinical<br />
study of sulphasalazine tablets, M.Sc.<br />
thesis College of Pharmacy, University of<br />
Baghdad, 1999.<br />
30. Sandborn WJ, Tremaine WJ, Offerd KP,<br />
Transdermal nicotine for mildly to<br />
moderately active ulcerative colitis . A<br />
randomized double – blind , placebo-<br />
controlled trial . Ann Intern Med :1997;<br />
126: 364 -71.