Chemical Weathering and Soil Development
Chemical Weathering and Soil Development
Chemical Weathering and Soil Development
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Chemical</strong> <strong>Weathering</strong><br />
<strong>and</strong><br />
<strong>Soil</strong> <strong>Development</strong><br />
No new<br />
minerals<br />
Yakov Kuzyakov<br />
Ökopedologie<br />
der Gemäßigten Zonen<br />
1. Types of weathering<br />
2. Main chemical weathering processes<br />
3. Factors affecting chemical weathering<br />
4. Mineral stability <strong>and</strong> weathering<br />
5. Mineral transformation<br />
6. <strong>Soil</strong> development<br />
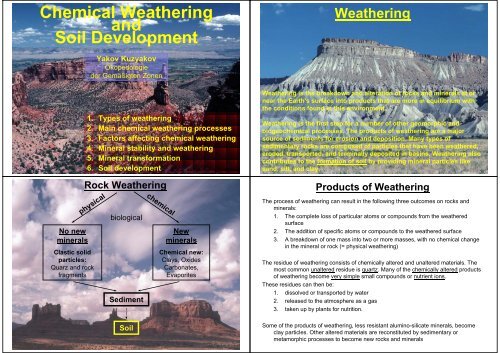
Rock <strong>Weathering</strong><br />
physical<br />
Clastic solid<br />
particles:<br />
Quarz <strong>and</strong> rock<br />
fragments<br />
biological<br />
Sediment<br />
<strong>Soil</strong><br />
chemical<br />
New<br />
minerals<br />
<strong>Chemical</strong> new:<br />
Clays, Oxides<br />
Carbonates,<br />
Evaporites<br />
<strong>Weathering</strong><br />
<strong>Weathering</strong> is the breakdown <strong>and</strong> alteration of rocks <strong>and</strong> minerals at or<br />
near the Earth's surface into products that are more in equilibrium with<br />
the conditions found in this environment.<br />
<strong>Weathering</strong> is the first step for a number of other geomorphic <strong>and</strong><br />
biogeochemical processes. The products of weathering are a major<br />
source of sediments for erosion <strong>and</strong> deposition. Many types of<br />
sedimentary rocks are composed of particles that have been weathered,<br />
eroded, transported, <strong>and</strong> terminally deposited in basins. <strong>Weathering</strong> also<br />
contributes to the formation of soil by providing mineral particles like<br />
s<strong>and</strong>, silt, <strong>and</strong> clay.<br />
Products of <strong>Weathering</strong><br />
The process of weathering can result in the following three outcomes on rocks <strong>and</strong><br />
minerals:<br />
1. The complete loss of particular atoms or compounds from the weathered<br />
surface<br />
2. The addition of specific atoms or compounds to the weathered surface<br />
3. A breakdown of one mass into two or more masses, with no chemical change<br />
in the mineral or rock (= physical weathering)<br />
The residue of weathering consists of chemically altered <strong>and</strong> unaltered materials. The<br />
most common unaltered residue is quartz. Many of the chemically altered products<br />
of weathering become very simple small compounds or nutrient ions.<br />
These residues can then be:<br />
1. dissolved or transported by water<br />
2. released to the atmosphere as a gas<br />
3. taken up by plants for nutrition.<br />
Some of the products of weathering, less resistant alumino-silicate minerals, become<br />
clay particles. Other altered materials are reconstituted by sedimentary or<br />
metamorphic processes to become new rocks <strong>and</strong> minerals
Physical weathering<br />
Types of physical weathering are:<br />
- Frost wedging<br />
- Expansion <strong>and</strong> contraction due to diurnal temperature changes<br />
- Release of overburden pressure -- (sheeting <strong>and</strong> exfoliation)<br />
- Biological process -- ie. rooting<br />
insolation (thermal) weathering<br />
expansion <strong>and</strong> contraction with wetting <strong>and</strong> drying<br />
the surface temperature of dark colored rock can vary from 0-50 o C between day <strong>and</strong><br />
night, since rock (especially jointed rock) has low thermal conductivity<br />
the differential stresses of expansion <strong>and</strong> contraction of the outer 1-5 cm of rock<br />
causes separation of concentric shallow layers called spalling or spheroidal weathering<br />
in boulders<br />
frost shattering<br />
the force of water in rock fractures as it freezes <strong>and</strong> exp<strong>and</strong>s, or is forced into the rock<br />
by the pressure of freezing water<br />
the most common physical weathering process, given the widespread distribution of<br />
frost (even in the tropic at high elevations)<br />
most effective in coastal arctic <strong>and</strong> alpine environments where there are hundreds of<br />
frost cycles per year<br />
the specific volume (vol./unit mass) of water increases by 9% upon freezing producing<br />
stress that is greater than the strength of all common rock<br />
<strong>Chemical</strong> weathering<br />
Alteration to cause chemical or mineralogical<br />
changes: weakens rocks<br />
The reactive components in the Earth's atmosphere<br />
include:<br />
– Oxygen O 2<br />
– Carbon Dioxide - CO 2<br />
–Water -H 2 O<br />
Nitrogen, the most abundant consistent in the atmosphere<br />
has little effect on the chemical weathering of rocks <strong>and</strong><br />
minerals.
Main schema of chemical weathering<br />
Hydrolysis<br />
mineral cations (e.g., Ca + , Fe + , Na + , K + , Al + ) are replaced by<br />
hydrogen ions (H + ) from acidic water<br />
Mg 2 SiO 4 (Olivine) + 4 H + 2 Mg 2+ + H 4 SiO 4<br />
KAlSi 3 O 2 (Feldspar) + H + Al 2 Si 2 O 5 (OH) 4 (Kaolinite) + K + + H 4 SiO 4<br />
• the most common weathering process<br />
• pure water is a poor H + donor, however biogenic CO 2<br />
dissolves in water to produce carbonic acid:<br />
• the weathering products are in solution or a residue is clay,<br />
that is, the first stage of soil development<br />
• the soil water solution becomes more basic as H + is<br />
consumed<br />
Hydrolysis is the weathering reaction that occurs when the two<br />
surfaces of water <strong>and</strong> compound meet. It involves the reaction<br />
between mineral ions <strong>and</strong> the ions of water (OH - <strong>and</strong> H + ), <strong>and</strong><br />
results in the decomposition of the rock surface by forming new<br />
compounds, <strong>and</strong> by increasing the pH of the solution involved<br />
through the release of the hydroxide ions.<br />
Hydrolysis is especially effective in the weathering of common silicate<br />
<strong>and</strong> alumino-silicate minerals because of their electrically charged<br />
crystal surfaces.<br />
Muscovite mica<br />
K 2 Al 6 Si 6 O 20 (OH) 4<br />
↓<br />
Illite clay<br />
KAl 5 Si 7 O 20(OH) 4<br />
↓<br />
Kaolinite clay<br />
Al 4 Si 4 O 10 (OH) 8<br />
↓<br />
Gibbsite<br />
Al(OH) 3<br />
(Bauxite)<br />
Hydrolysis<br />
involves the<br />
progressive loss<br />
of the more<br />
soluble cations<br />
(Na, K, Ca, Mg)<br />
<strong>and</strong> under<br />
extreme<br />
conditions Si<br />
The most common<br />
chemical weathering processes<br />
• Hydrolysis<br />
• Oxidation<br />
• Reduction<br />
• Hydration<br />
• Carbonation<br />
• Chelation/Complexation<br />
• Solution<br />
Oxidation <strong>and</strong> Reduction<br />
Oxidation is loss of an electron (e - ) to dissolved oxygen<br />
- Fe is the most commonly oxidized mineral element:<br />
Fe 2+ (ferrous iron) Fe 3+ (ferric iron)<br />
8 FeO + 2 O 2 4 Fe 2 O 3<br />
4 FeSiO 3 + O 2 2Fe 2O 3 (hematite) + 4 SiO 2 (aq)<br />
2 FeS 2 (pyrite) + O 2 Fe 2O 3 (hematite) + 2 S<br />
4FeS 2 (pyrite) + 15 O 2 +14H 2 O-><br />
4Fe(OH) 3 (Limonite) + 8 H 2 SO 4<br />
- other readily oxidized mineral elements include Mg, S, Cr<br />
Oxidation is the reaction that occurs between compounds<br />
<strong>and</strong> O 2. The net result of this reaction is the removal of one<br />
or more e - from a compound, which causes the structure to<br />
be less rigid <strong>and</strong> increasingly unstable. The most common<br />
oxides are those of Fe <strong>and</strong> Al, <strong>and</strong> their respective red <strong>and</strong><br />
yellow staining of soils is quite common in tropical regions<br />
which have high temperatures <strong>and</strong> precipitation.<br />
Atmospheric O 2 is the most abund<strong>and</strong> oxidizing agent on<br />
Earth, <strong>and</strong> has been since about 1.8 billion years.<br />
Reduction is the reverse of oxidation: is thus caused by the<br />
addition of one or more e - resulting in more stable products
Acidic sulfate weathering<br />
in a Sulfudept forming<br />
from pyritic main spoil.<br />
A dark sulfidic horizon<br />
underlines a lighted<br />
colored sulfuric horizon.<br />
Oxidation of sulfides may<br />
produce acidic drainage<br />
waters. (10 cm bars)<br />
Carbonation<br />
dissolving of CaCO 3 (limestone) in acidic groundwater<br />
- similar to hydrolysis but the all the products are ionic, there is<br />
no residue<br />
- bicarbonate (HCO 3 - ) is a product of carbonation <strong>and</strong> a major<br />
part of the dissolved load of most rivers<br />
- the carbonation of limestone results in karst topography:<br />
caves, sinkholes, etc.<br />
CaCO 3 + H 2CO 3 Ca 2+ + 2 HCO 3 -<br />
Solubility of CaCO 3 = 0.01 g/l (pure H 2O) – 0.3 g/l (H 2O+CO 2 sat)<br />
Carbonation is the reaction of CO 3 2- ) <strong>and</strong> HCO3 - ions with<br />
minerals. The formation of carbonates usually takes place as<br />
a result of other chemical processes.<br />
Carbonation is especially active when<br />
the reaction environment is<br />
abundant with CO 2. The formation<br />
of carbonic acid (H 2CO 3) is<br />
important in the solution of<br />
carbonates <strong>and</strong> the decomposition<br />
of mineral surfaces because of its<br />
acidic nature<br />
Hydration<br />
Hydration involves the rigid attachment of H + <strong>and</strong> OH - ions to a<br />
reacted compound. In many situations the H + <strong>and</strong> OH - ions<br />
become a structural part of the crystal lattice of the mineral<br />
CaSO 4 + 2 H 2O CaSO 4 ·2 H 2O<br />
Anhydrid Gyps<br />
Dehydration (=reverse process)<br />
2Fe(OH) 3 (Limonite, orange) Fe 2 O 3 (Hematite, red) +3H 2 O<br />
Hydration also allows for the acceleration of other<br />
decompositional reactions by exp<strong>and</strong>ing the crystal lattice<br />
offering more surface area for reaction.<br />
Chelation / Complexation<br />
bonding of mineral cations <strong>and</strong> organic molecules produced by plants<br />
• these chelates are stable at a pH at which the cation would normally<br />
precipitate <strong>and</strong> thus they are leached in seeping soil water<br />
• H + released during chelation from organic molecules is available for<br />
hydrolysis<br />
• thus plants, such as the lichens on bare rocks, contribute to the<br />
decomposition of soil <strong>and</strong> rock<br />
K 2 (Si 6 Al 2)Al 4 O 20 (OH) 4 (muscovite) + 6 C 2 O 4 2- (oxalat) + 2 0H - <br />
6[Al(C 2 O 4 )] + (aq) + 6 H 4 SiO 4 + 2 K +
Solution<br />
Water <strong>and</strong> the ions it carries as it moves<br />
through <strong>and</strong> around rocks <strong>and</strong> minerals<br />
can further the weathering process<br />
Quarz (SiO 2 ) + H 2 O Silicic acid (H 2 SiO 3 )<br />
Molecules can mix in solution to form a great variety of basic <strong>and</strong><br />
acidic decompositional compounds. The extent, however, of<br />
rock being subjected to solution is determined primarily by<br />
climatic conditions. Solution tends to be most effective in areas<br />
that have humid <strong>and</strong> hot climates.<br />
CaCO 3 (s) + H 2 O Ca 2+ + HCO 3 - + OH -<br />
MgSiO 4 (s) + 4 CO 2 (aq) + 4 H 2 O 2Mg(HCO 3 ) 2 (aq) + H 4 SiO 4<br />
-O-M + H 2 O -O--M--OH 2 O 2- + M-OH + H +<br />
How would climate<br />
influence weathering<br />
rates at locations on<br />
the map?<br />
Weakest -O-M<br />
bonds will weather<br />
first<br />
Bond BE/kj/mol<br />
Ti - O 674<br />
Al - O 582<br />
Si - O 464<br />
Ca - O 423<br />
Mn - O 389<br />
Fe - O 389<br />
Mg - O 377<br />
1. Tropical rainforest (equatorial regions including South America, Africa,<br />
Indonesia, southeast Asia): chemical weathering rates would be rapid as<br />
these regions have both high temperatures <strong>and</strong> plenty of rainfall.<br />
2. Hot desert (subtropical regions including North Africa [Sahara], southwest<br />
South America [Atacama], southwest Africa [Namib], Asia [Gobi],<br />
southwestern U.S., central Australia): plenty of heat but insufficient water to<br />
cause significant physical <strong>and</strong>/or chemical weathering.<br />
3. Temperate mountains (Rocky Mountains, Sierra Nevada Mountains, Alps,<br />
Andes Mountains): insufficient temperatures for rapid chemical weathering<br />
but elevations contribute to freeze-thaw cycles necessary for ice wedging.<br />
4. Polar Regions (Alaska, Antarctica, Siberia): too much cold weather to<br />
permit thawing. Water in solid form (ice) unable to react with rock.<br />
Biological <strong>Weathering</strong><br />
Organisms can assist in breaking down rock<br />
into sediment or soil<br />
1. Roots of trees <strong>and</strong> other plants<br />
2. Lichens, fungi, <strong>and</strong> other micro-organisms<br />
3. Animals (including humans)<br />
Distribution of clay minerals
Factors affecting weathering<br />
• The most important factor affecting all chemical weathering processes is climate.<br />
Climatic conditions control the rate of weathering that takes place by regulating the<br />
catalysts of moisture <strong>and</strong> temperature. Tropical weathering rates (temperature <strong>and</strong><br />
moisture are at maximum) are 3-4 times higher than rates in temperate environments.<br />
– Arid (<strong>and</strong> cold) climate: mechanical weathering predominate<br />
– Humid warm climate: chemical weathering predominate<br />
• pH<br />
– Many surface & near surface<br />
terrestrial waters are acidic<br />
• Rain (pH=5.6)<br />
• Acid rain (pH~4.4) & fog<br />
– Ocean water is slightly alkaline<br />
Other factors<br />
•Rock/mineral type & distribution<br />
•Time<br />
•Drainage<br />
•Relief<br />
•Vegetation<br />
Rates of weathering<br />
• Highly variable depending on climate<br />
• <strong>Chemical</strong> weathering rates doubles with 10 °C<br />
• Mechanical weathering is very slow<br />
• <strong>Weathering</strong> rates vary through time<br />
(prior to terrestrial plants the wheathering was slower)<br />
• Surface area<br />
Rates of <strong>Weathering</strong> of Clean Rock Surfaces (micro-meters/1000years)<br />
Rock Type Cold Climate Warm, Humid Climate<br />
Basalt 10 100<br />
Granite 1 10<br />
Marble 20 200<br />
Zusammenhang:<br />
Klima – Vegetation –<br />
Nährstoffakkumulation<br />
24
Limitierende Faktoren:<br />
Temperatur (T), Wasser (W), Nährstoffe (NS)<br />
T, W T, NS T, NS W W, (NS) (W) NS (W)<br />
P, ... N, P, N, P, Ca, Mg, S,<br />
Ca, Mg,<br />
B, Mo<br />
B, Mo<br />
+ P, (N),<br />
MikroNS<br />
Wheathering of minerals<br />
+ P, Ca, Mg, S,<br />
B, Cl, Zn,<br />
Cu, Mo<br />
Mineral <strong>and</strong> chemical composition are important factors as to the extent to<br />
which the stone will be affected <strong>and</strong> the type of effects it may display.<br />
Generally...<br />
• Granite-type stones are more resistant to the mechanical processes with<br />
the exception of salt decay <strong>and</strong> more susceptible to the chemical<br />
processes of hydrolysis <strong>and</strong> in some cases oxidation<br />
• Limestone <strong>and</strong> marble are vulnerable to salt decay, dissolution, hydration<br />
<strong>and</strong> in some cases oxidation<br />
• S<strong>and</strong>stone is susceptible to the processes of salt decay, oxidation <strong>and</strong> if it<br />
is a calcareous variety of s<strong>and</strong>stone, it is vulnerable to the dissolution<br />
• Clay slates are vulnerable to the chemical processes of hydration,<br />
hydrolysis <strong>and</strong> some varieties are affected by the oxidation<br />
25<br />
<strong>Weathering</strong><br />
events<br />
Periods with elevated<br />
CO 2 should have<br />
evidence for<br />
enhanced<br />
weathering: (e.g.,<br />
Cretaceous, ...)<br />
Change in humidity<br />
(e.g., Miocene ca.<br />
24-5 billion years)<br />
Rise of O 2 above a<br />
threshold that<br />
allowed onset of<br />
global atmospheric<br />
oxidation<br />
(Precambian ca.<br />
1.8 billion years)<br />
Most rocks are composed of minerals (ordered arrangements of atoms)<br />
• There are thous<strong>and</strong>s of minerals, but only a few compose the bulk of most rocks.<br />
• The ultimate source of rocky material is magma (molten rock) from within the<br />
earth.<br />
Eruption of magma on the surface (lava) forms fine-grained extrusive rocks:<br />
• Rhyolites (rich in Si,Al <strong>and</strong> K)<br />
• Basalts (rich in Mg <strong>and</strong> Fe)<br />
When deeply-buried magmas solidify slowly, they make coarse-grained plutonic rocks:<br />
• Granite, the coarse-grained equivalent to rhyolite<br />
• Gabbro, the coarse-grained equivalent to basalt<br />
The most common elements in basalts <strong>and</strong> gabbro (given as oxides):<br />
Oxide<br />
Weight % in Basalt<br />
(or gabbro)<br />
Weight % in Rhyolite<br />
(or granite)<br />
SiO2 50.83 72.66<br />
Al2O3 14.07 13.45<br />
K2O 0.82 5.35<br />
Na2O 2.23 2.99<br />
FeO <strong>and</strong> Fe2O3 11.93 2.00<br />
CaO 10.42 1.13
Factors affecting stability<br />
of parent material minerals<br />
1. Resistance of ingneous minerals to weathering is<br />
the same as the order of crystalization from<br />
cooling magmas<br />
2. Positions of ions in the structure of feldspars:<br />
tetraedra of Al 3+ > Si 4+ // K + > Na + > Ca 2+<br />
3. Tetraedra linkage to each other<br />
4. Fe 2+ <strong>and</strong> Mn 2+ content<br />
Differential <strong>Weathering</strong><br />
Not all rock types are equally affected by these differing weathering reactions. Instead,<br />
it will depend on the type of minerals in the particular rock. Most of the minerals<br />
(especially the silicates) form at very high temperatures <strong>and</strong> some of them at very<br />
high pressures. That means that these minerals are stable (happy) at these high<br />
temperatures <strong>and</strong> pressure. If you bring these minerals to conditions that are quite<br />
different (such as the temperature <strong>and</strong> pressure conditions at the Earth's surface),<br />
these minerals will not longer be stable. A mineral that is unstable is likely to be<br />
more easily weathered.<br />
The higher the temperature <strong>and</strong> pressure of formation, the more unstable the mineral<br />
is. That means that the group of minerals we've designated the “high temperature”<br />
minerals will be weathered more quickly than those in the “low temperature”<br />
category. So what does this mean for the rocks that contain these minerals? Well,<br />
the mafic rocks contain the high temperature minerals, <strong>and</strong> therefore will weather<br />
more quickly than the felsic rocks, which contain the low temperature minerals. So,<br />
if an area of basalt is right next to an area of granite, after years of weathering, the<br />
basalt will weather away more quickly <strong>and</strong> become a valley, while the granite will be<br />
more resistant to weathering <strong>and</strong> will look like a hill compared to the basalt right<br />
next to it. If limestone will be added to the area, granite will weather even faster<br />
than basalt, because the rock is actually dissolved away, leaving no solid products.<br />
These differences in weathering rates are referred to as differential weathering.
Example: Feldspar weathering<br />
2 KAlSi 3 O 8 + 2 H 2 CO 3 + H 2 O Al 2 Si 2 O 5 (OH) 4 + 4 SiO 2 (aq) + 2 K + + 2 HCO 3 -<br />
spar + carbonic acid + water clay (with high water content) + dissolved ions<br />
• Start with feldspar end up with clay plus dissolved ions (Framework silicate -> sheet<br />
silicate plus ions)<br />
• Some of the water is absorbed by the clay (hydration)<br />
• Dissolved ions transported away<br />
• The dissolved silica again becomes important as a cement<br />
• Several other minerals weather to form clays such as amphiboles <strong>and</strong> micas<br />
• Other minerals, such as olivine <strong>and</strong> pyroxene (top of Bowens' reaction series) are<br />
so unstable they often dissolve completely)<br />
One of the most stable minerals is quartz (bottom of Bowen's reaction Series)<br />
• major component in beach s<strong>and</strong>s - is often the only mineral left<br />
• Resistant to weathering for 2 reasons:<br />
– very stable (Bowen again)<br />
– lacks cations that can be easily substituted by H +
Generilized schema of mineral weathering to clay,<br />
<strong>and</strong> clay transformations<br />
Primary minerals weather to form clays that weather to solutes <strong>and</strong> other clays<br />
<strong>Chemical</strong><br />
weathering on<br />
granite<br />
Unweathered granite contains these minerals:<br />
• Na Plagioclase feldspar<br />
• K feldspar<br />
• Quartz<br />
• Lesser amounts of biotite, amphibole, or muscovite<br />
1.The feldspars will undergo hydrolysis to form kaolinite<br />
(clay) <strong>and</strong> Na <strong>and</strong> K ions<br />
2.The Na <strong>and</strong> K ions will be removed through leaching<br />
3.The biotite <strong>and</strong>/or amphibole will undergo hydrolysis to<br />
form clay, <strong>and</strong> oxidation to form iron oxides<br />
4.The quartz (<strong>and</strong> muscovite, if present) will remain as<br />
residual minerals because they are very resistant to<br />
weathering
Schematic presentation of basic <strong>and</strong> acidic zones through soil during development<br />
(this sequence also represents soil profiles from arid to humid to humid tropical regions)<br />
Slightly<br />
weathered<br />
Organic<br />
Neutral to<br />
slightly alkaline<br />
Slightly<br />
alkaline<br />
CaCO 3<br />
accumulation<br />
Soluble salt<br />
accumulation<br />
Parent<br />
material<br />
<strong>Weathering</strong> <strong>and</strong> soil development<br />
Organic<br />
Slightly acid<br />
Neutral to<br />
slightly alkaline<br />
Parent<br />
material<br />
Moderately weathered<br />
Organic<br />
Highly acid<br />
Acid<br />
Parent<br />
material<br />
Strongly<br />
weathered<br />
Organic<br />
Neutral to<br />
slightly acid<br />
Highly acid<br />
Parent<br />
material<br />
Sequence of clay mineral distribution with<br />
increasing soil development<br />
Relat. degree of Prominent minerals in soil clay fraction<br />
soil development<br />
1. Gypsum, sulfides, <strong>and</strong> soluble salts<br />
2. Calcite, dolomite, <strong>and</strong> apatite<br />
3. Olivine, amphiboles, <strong>and</strong> pyroxenes<br />
4. Micas <strong>and</strong> clorite<br />
5. Feldspars<br />
6. Quartz<br />
7. Muscovite<br />
8. Vermiculite <strong>and</strong> hydrous micas<br />
9. Montmorillonites<br />
10. Kaolinite <strong>and</strong> halloisite<br />
11. Gibbsite <strong>and</strong> allophane<br />
12. Goetite, limonite, <strong>and</strong> hematite<br />
13. Titanium oxides, zircon, <strong>and</strong> corrundum<br />
Effects of chemical weathering<br />
• Weaken coherence between mineral grains,<br />
so rock crumbles<br />
• Forms solutions, which can be removed.<br />
So rocks crumbles more<br />
• Clay forms, increase volume of weathered material.<br />
So weathered rocks pulls away <strong>and</strong> exposes fresh rock.<br />
So more weathering occurs
Summary<br />
• <strong>Weathering</strong> is a prerequisite for soil formation<br />
• Types of weathering<br />
–Physical<br />
– <strong>Chemical</strong>: Hydrolysis, Oxidation, Reduction, Hydration, Carbonation,<br />
Chelation/Complexation, Solution<br />
– Biological<br />
• Factors<br />
– Climate: Temperature, Precipitation<br />
–pH<br />
– Mineral structure<br />
– Formation conditions<br />
– <strong>Chemical</strong> composition<br />
• <strong>Weathering</strong> rates vary between years <strong>and</strong> billions of years