CA Animal Rabies Vaccine Compendium, 2012 - California ...
CA Animal Rabies Vaccine Compendium, 2012 - California ...
CA Animal Rabies Vaccine Compendium, 2012 - California ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
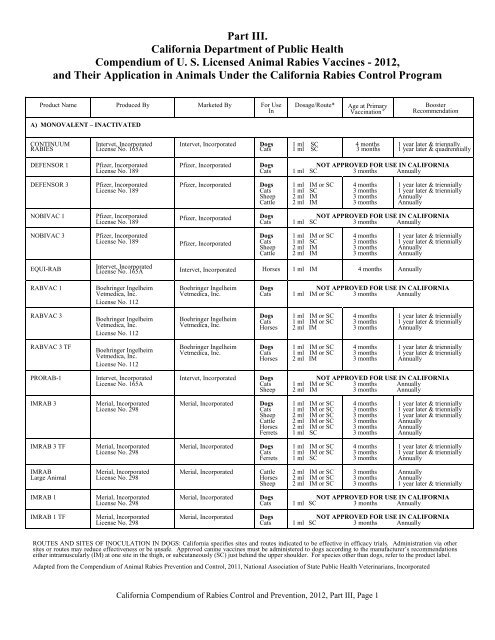
Part III.<br />
<strong>California</strong> Department of Public Health<br />
<strong>Compendium</strong> of U. S. Licensed <strong>Animal</strong> <strong>Rabies</strong> <strong>Vaccine</strong>s - <strong>2012</strong>,<br />
and Their Application in <strong>Animal</strong>s Under the <strong>California</strong> <strong>Rabies</strong> Control Program<br />
Product Name<br />
Produced By<br />
A) MONOVALENT – INACTIVATED<br />
CONTINUUM<br />
RABIES<br />
DEFENSOR 1<br />
DEFENSOR 3<br />
NOBIVAC 1<br />
NOBIVAC 3<br />
EQUI-RAB<br />
RABVAC 1<br />
RABVAC 3<br />
RABVAC 3 TF<br />
PRORAB-1<br />
IMRAB 3<br />
IMRAB 3 TF<br />
IMRAB<br />
Large <strong>Animal</strong><br />
IMRAB 1<br />
IMRAB 1 TF<br />
Intervet, Incorporated<br />
License No. 165A<br />
Pfizer, Incorporated<br />
License No. 189<br />
Pfizer, Incorporated<br />
License No. 189<br />
Pfizer, Incorporated<br />
License No. 189<br />
Marketed By For Use<br />
In<br />
Intervet, Incorporated Dogs<br />
Cats<br />
Pfizer, Incorporated Dogs<br />
Cats<br />
Pfizer, Incorporated Dogs<br />
Cats<br />
Sheep<br />
Cattle<br />
Pfizer, Incorporated<br />
Pfizer, Incorporated<br />
License No. 189 Pfizer, Incorporated<br />
Dogs<br />
Cats<br />
Dogs<br />
Cats<br />
Sheep<br />
Cattle<br />
Dosage/Route*<br />
1 ml SC<br />
1 ml SC<br />
Age at Primary<br />
Vaccination <br />
4 months<br />
3 months<br />
<strong>California</strong> <strong>Compendium</strong> of <strong>Rabies</strong> Control and Prevention, <strong>2012</strong>, Part III, Page 1<br />
Booster<br />
Recommendation<br />
1 year later & triennally<br />
1 year later & quadrennially<br />
NOT APPROVED FOR USE IN <strong>CA</strong>LIFORNIA<br />
1 ml SC 3 months Annually<br />
1 ml IM or SC<br />
1 ml SC<br />
2 ml IM<br />
2 ml IM<br />
4 months<br />
3 months<br />
3 months<br />
3 months<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
Annually<br />
NOT APPROVED FOR USE IN <strong>CA</strong>LIFORNIA<br />
1 ml SC 3 months Annually<br />
1 ml IM or SC<br />
1 ml SC<br />
2 ml IM<br />
2 ml IM<br />
4 months<br />
3 months<br />
3 months<br />
3 months<br />
Intervet, Incorporated<br />
License No. 165A Intervet, Incorporated Horses 1 ml IM 4 months Annually<br />
Boehringer Ingelheim<br />
Vetmedica, Inc.<br />
License No. 112<br />
Boehringer Ingelheim<br />
Vetmedica, Inc.<br />
License No. 112<br />
Boehringer Ingelheim<br />
Vetmedica, Inc.<br />
License No. 112<br />
Intervet, Incorporated<br />
License No. 165A<br />
Merial, Incorporated<br />
License No. 298<br />
Merial, Incorporated<br />
License No. 298<br />
Merial, Incorporated<br />
License No. 298<br />
Merial, Incorporated<br />
License No. 298<br />
Merial, Incorporated<br />
License No. 298<br />
Boehringer Ingelheim<br />
Vetmedica, Inc.<br />
Boehringer Ingelheim<br />
Vetmedica, Inc.<br />
Boehringer Ingelheim<br />
Vetmedica, Inc.<br />
Dogs<br />
Cats<br />
Dogs<br />
Cats<br />
Horses<br />
Dogs<br />
Cats<br />
Horses<br />
Intervet, Incorporated Dogs<br />
Cats<br />
Sheep<br />
Merial, Incorporated Dogs<br />
Cats<br />
Sheep<br />
Cattle<br />
Horses<br />
Ferrets<br />
Merial, Incorporated Dogs<br />
Cats<br />
Ferrets<br />
Merial, Incorporated Cattle<br />
Horses<br />
Sheep<br />
Merial, Incorporated Dogs<br />
Cats<br />
Merial, Incorporated Dogs<br />
Cats<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
Annually<br />
NOT APPROVED FOR USE IN <strong>CA</strong>LIFORNIA<br />
1 ml IM or SC 3 months Annually<br />
1 ml IM or SC<br />
1 ml IM or SC<br />
2 ml IM<br />
1 ml IM or SC<br />
1 ml IM or SC<br />
2 ml IM<br />
4 months<br />
3 months<br />
3 months<br />
4 months<br />
3 months<br />
3 months<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
NOT APPROVED FOR USE IN <strong>CA</strong>LIFORNIA<br />
1 ml IM or SC 3 months Annually<br />
2 ml IM 3 months Annually<br />
1 ml IM or SC<br />
1 ml IM or SC<br />
2 ml IM or SC<br />
2 ml IM or SC<br />
2 ml IM or SC<br />
1 ml SC<br />
1 ml IM or SC<br />
1 ml IM or SC<br />
1 ml SC<br />
2 ml IM or SC<br />
2 ml IM or SC<br />
2 ml IM or SC<br />
4 months<br />
3 months<br />
3 months<br />
3 months<br />
3 months<br />
3 months<br />
4 months<br />
3 months<br />
3 months<br />
3 months<br />
3 months<br />
3 months<br />
1 year later & triennially<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
Annually<br />
Annually<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
Annually<br />
Annually<br />
1 year later & triennially<br />
NOT APPROVED FOR USE IN <strong>CA</strong>LIFORNIA<br />
1 ml SC 3 months Annually<br />
NOT APPROVED FOR USE IN <strong>CA</strong>LIFORNIA<br />
1 ml SC 3 months Annually<br />
ROUTES AND SITES OF INOCULATION IN DOGS: <strong>California</strong> specifies sites and routes indicated to be effective in efficacy trials. Administration via other<br />
sites or routes may reduce effectiveness or be unsafe. Approved canine vaccines must be administered to dogs according to the manufacturer’s recommendations<br />
either intramuscularly (IM) at one site in the thigh, or subcutaneously (SC) just behind the upper shoulder. For species other than dogs, refer to the product label.<br />
Adapted from the <strong>Compendium</strong> of <strong>Animal</strong> <strong>Rabies</strong> Prevention and Control, 2011, National Association of State Public Health Veterinarians, Incorporated
- Continued -<br />
Part III.<br />
<strong>California</strong> Department of Health Services<br />
<strong>Compendium</strong> of U. S. Licensed <strong>Animal</strong> <strong>Rabies</strong> <strong>Vaccine</strong>s - <strong>2012</strong>,<br />
and Their Application in <strong>Animal</strong>s Under the <strong>California</strong> <strong>Rabies</strong> Control Program<br />
Product Name<br />
Produced By<br />
Marketed By For Use<br />
In<br />
B) MONOVALENT - RABIES GLYCOPROTEIN, LIVE <strong>CA</strong>NARY POX VECTOR<br />
PUREVAX Feline<br />
<strong>Rabies</strong><br />
Merial, Incorporated<br />
License No. 298<br />
C) COMBINATION - INACTIVATED RABIES<br />
CONTINUUM<br />
DAP-R<br />
CONTINUUM<br />
Feline HCP-R<br />
EQUINE<br />
POTOMAVAC +<br />
IMRAB<br />
Intervet, Incorporated<br />
License No. 165A<br />
Intervet, Incorporated<br />
License No. 165A<br />
Merial, Incorporated<br />
License No. 298<br />
Merial, Incorporated Cats 1 ml SC<br />
D) COMBINATION - RABIES GLYCOPROTEIN, LIVE <strong>CA</strong>NARY POX VECTOR<br />
PUREVAX FELINE<br />
3/ RABIES<br />
PUREVAX FELINE<br />
4/ RABIES<br />
Merial, Incorporated<br />
License No. 298<br />
Merial, Incorporated<br />
License No. 298<br />
* Intramuscularly (IM) at one site in the thigh.<br />
Subcutaneously (SC) just behind the upper shoulder.<br />
Minimum age (or older) and revaccinated one year later. A month = 28 days.<br />
Dosage/Route* Age at Primary<br />
Vaccination <br />
Intervet, Incorporated Dogs 1 ml SC 4 months<br />
Intervet, Incorporated Cats 1 ml SC 3 months<br />
Merial, Incorporated Horses 1 ml IM 3 months<br />
Merial, Incorporated Cats 1 ml SC 8 weeks<br />
3 months<br />
Merial, Incorporated Cats 1 ml SC 8 weeks<br />
<strong>California</strong> <strong>Compendium</strong> of <strong>Rabies</strong> Control and Prevention, <strong>2012</strong>, Part III, Page 2<br />
3 months Annually<br />
3 months<br />
Booster<br />
Recommendation<br />
1 year later & triennially<br />
1 year later & triennially<br />
Annually<br />
Every 3 weeks until 3<br />
months & annually<br />
3 weeks later & annually<br />
Every 3 weeks until 3<br />
months & annually<br />
3 weeks later & annually