KVÄLLSSYMPOSIUM 2008 Vaccinering av hund och katt
KVÄLLSSYMPOSIUM 2008 Vaccinering av hund och katt
KVÄLLSSYMPOSIUM 2008 Vaccinering av hund och katt
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Table 1<br />
Titer of Challenge Virus Standard (CVS-11) and Flury low egg passage<br />
(Flury-LEP), the two rabies virus strains used as the challenge viruses<br />
for each serological testing group<br />
Serological testing group Titer of CVS-11 Titer of Flury-LEP<br />
1 42.1 41.1<br />
2 40.0 41.2<br />
3 41.0 40.0<br />
4 41.4 42.3<br />
5 41.1 40.9<br />
Geometric mean 41.1 41.1<br />
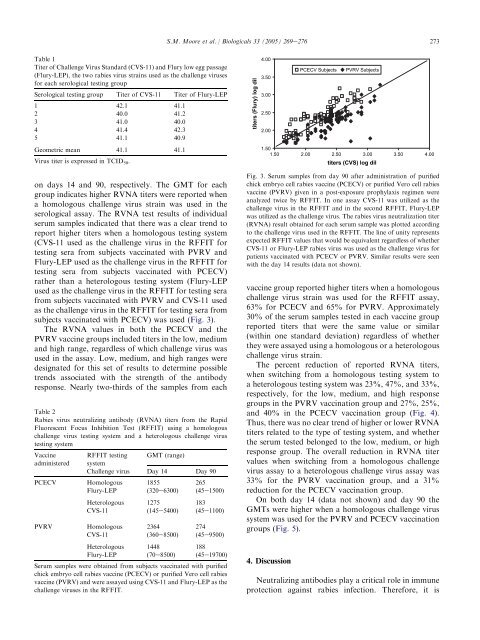
Virus titer is expressed in TCID50. on days 14 and 90, respectively. The GMT for each<br />
group indicates higher RVNA titers were reported when<br />
a homologous challenge virus strain was used in the<br />
serological assay. The RVNA test results of individual<br />
serum samples indicated that there was a clear trend to<br />
report higher titers when a homologous testing system<br />
(CVS-11 used as the challenge virus in the RFFIT for<br />
testing sera from subjects vaccinated with PVRV and<br />
Flury-LEP used as the challenge virus in the RFFIT for<br />
testing sera from subjects vaccinated with PCECV)<br />
rather than a heterologous testing system (Flury-LEP<br />
used as the challenge virus in the RFFIT for testing sera<br />
from subjects vaccinated with PVRV and CVS-11 used<br />
as the challenge virus in the RFFIT for testing sera from<br />
subjects vaccinated with PCECV) was used (Fig. 3).<br />
The RVNA values in both the PCECV and the<br />
PVRV vaccine groups included titers in the low, medium<br />
and high range, regardless of which challenge virus was<br />
used in the assay. Low, medium, and high ranges were<br />
designated for this set of results to determine possible<br />
trends associated with the strength of the antibody<br />
response. Nearly two-thirds of the samples from each<br />
Table 2<br />
Rabies virus neutralizing antibody (RVNA) titers from the Rapid<br />
Fluorescent Focus Inhibition Test (RFFIT) using a homologous<br />
challenge virus testing system and a heterologous challenge virus<br />
testing system<br />
Vaccine RFFIT testing GMT (range)<br />
administered system<br />
Challenge virus Day 14 Day 90<br />
PCECV Homologous 1855 265<br />
Flury-LEP (320e6300) (45e1500)<br />
Heterologous 1275 183<br />
CVS-11 (145e5400) (45e1100)<br />
PVRV Homologous 2364 274<br />
CVS-11 (360e8500) (45e9500)<br />
Heterologous 1448 188<br />
Flury-LEP (70e8500) (45e19700)<br />
Serum samples were obtained from subjects vaccinated with purified<br />
chick embryo cell rabies vaccine (PCECV) or purified Vero cell rabies<br />
vaccine (PVRV) and were assayed using CVS-11 and Flury-LEP as the<br />
challenge viruses in the RFFIT.<br />
S.M. Moore et al. / Biologicals 33 (2005) 269e276<br />
titers (Flury) log dil<br />
4.00<br />
3.50<br />
3.00<br />
2.50<br />
2.00<br />
1.50<br />
1.50 2.00 2.50 3.00 3.50 4.00<br />
vaccine group reported higher titers when a homologous<br />
challenge virus strain was used for the RFFIT assay,<br />
63% for PCECV and 65% for PVRV. Approximately<br />
30% of the serum samples tested in each vaccine group<br />
reported titers that were the same value or similar<br />
(within one standard deviation) regardless of whether<br />
they were assayed using a homologous or a heterologous<br />
challenge virus strain.<br />
The percent reduction of reported RVNA titers,<br />
when switching from a homologous testing system to<br />
a heterologous testing system was 23%, 47%, and 33%,<br />
respectively, for the low, medium, and high response<br />
groups in the PVRV vaccination group and 27%, 25%,<br />
and 40% in the PCECV vaccination group (Fig. 4).<br />
Thus, there was no clear trend of higher or lower RVNA<br />
titers related to the type of testing system, and whether<br />
the serum tested belonged to the low, medium, or high<br />
response group. The overall reduction in RVNA titer<br />
values when switching from a homologous challenge<br />
virus assay to a heterologous challenge virus assay was<br />
33% for the PVRV vaccination group, and a 31%<br />
reduction for the PCECV vaccination group.<br />
On both day 14 (data not shown) and day 90 the<br />
GMTs were higher when a homologous challenge virus<br />
system was used for the PVRV and PCECV vaccination<br />
groups (Fig. 5).<br />
4. Discussion<br />
PCECV Subjects PVRV Subjects<br />
titers (CVS) log dil<br />
Fig. 3. Serum samples from day 90 after administration of purified<br />
chick embryo cell rabies vaccine (PCECV) or purified Vero cell rabies<br />
vaccine (PVRV) given in a post-exposure prophylaxis regimen were<br />
analyzed twice by RFFIT. In one assay CVS-11 was utilized as the<br />
challenge virus in the RFFIT and in the second RFFIT, Flury-LEP<br />
was utilized as the challenge virus. The rabies virus neutralization titer<br />
(RVNA) result obtained for each serum sample was plotted according<br />
to the challenge virus used in the RFFIT. The line of unity represents<br />
expected RFFIT values that would be equivalent regardless of whether<br />
CVS-11 or Flury-LEP rabies virus was used as the challenge virus for<br />
patients vaccinated with PCECV or PVRV. Similar results were seen<br />
with the day 14 results (data not shown).<br />
Neutralizing antibodies play a critical role in immune<br />
protection against rabies infection. Therefore, it is<br />
273