A simple guide for travel vaccines – compiled by Jane Chiodini
A simple guide for travel vaccines – compiled by Jane Chiodini
A simple guide for travel vaccines – compiled by Jane Chiodini
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
A <strong>simple</strong> <strong>guide</strong> <strong>for</strong> <strong>travel</strong> <strong>vaccines</strong> <strong>–</strong> <strong>compiled</strong> <strong>by</strong> <strong>Jane</strong> <strong>Chiodini</strong><br />
Always use this table in conjunction with in<strong>for</strong>mation from the SPC at www.medicines.org.uk,<br />
the BNF at www.bnf.org and the DH Green Book at http://immunisation.dh.gov.uk/<br />
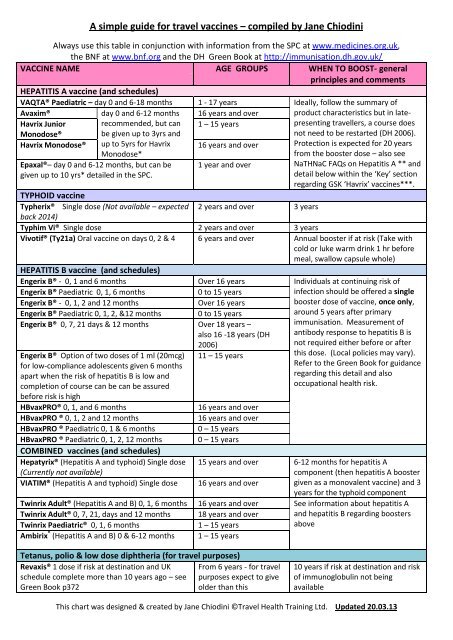
VACCINE NAME AGE GROUPS WHEN TO BOOST- general<br />
principles and comments<br />
HEPATITIS A vaccine (and schedules)<br />
VAQTA® Paediatric <strong>–</strong> day 0 and 6-18 months 1 - 17 years Ideally, follow the summary of<br />
Avaxim®<br />
day 0 and 6-12 months 16 years and over product characteristics but in late-<br />
Havrix Junior recommended, but can 1 <strong>–</strong> 15 years<br />
presenting <strong>travel</strong>lers, a course does<br />
Monodose®<br />
be given up to 3yrs and<br />
not need to be restarted (DH 2006).<br />
Havrix Monodose® up to 5yrs <strong>for</strong> Havrix 16 years and over Protection is expected <strong>for</strong> 20 years<br />
Monodose*<br />
from the booster dose <strong>–</strong> also see<br />
Epaxal®<strong>–</strong> day 0 and 6-12 months, but can be 1 year and over<br />
NaTHNaC FAQs on Hepatitis A ** and<br />
given up to 10 yrs* detailed in the SPC.<br />
TYPHOID vaccine<br />
detail below within the ‘Key’ section<br />
regarding GSK ‘Havrix’ <strong>vaccines</strong>***.<br />
Typherix® Single dose (Not available <strong>–</strong> expected<br />
back 2014)<br />
2 years and over 3 years<br />
Typhim Vi® Single dose 2 years and over 3 years<br />
Vivotif® (Ty21a) Oral vaccine on days 0, 2 & 4<br />
HEPATITIS B vaccine (and schedules)<br />
6 years and over Annual booster if at risk (Take with<br />
cold or luke warm drink 1 hr be<strong>for</strong>e<br />
meal, swallow capsule whole)<br />
Engerix B® - 0, 1 and 6 months Over 16 years Individuals at continuing risk of<br />
Engerix B® Paediatric 0, 1, 6 months 0 to 15 years<br />
infection should be offered a single<br />
Engerix B® - 0, 1, 2 and 12 months Over 16 years<br />
booster dose of vaccine, once only,<br />
Engerix B® Paediatric 0, 1, 2, &12 months 0 to 15 years<br />
around 5 years after primary<br />
Engerix B® 0, 7, 21 days & 12 months<br />
Engerix B® Option of two doses of 1 ml (20mcg)<br />
<strong>for</strong> low-compliance adolescents given 6 months<br />
apart when the risk of hepatitis B is low and<br />
completion of course can be can be assured<br />
be<strong>for</strong>e risk is high<br />
Over 18 years <strong>–</strong><br />
also 16 -18 years (DH<br />
2006)<br />
11 <strong>–</strong> 15 years<br />
immunisation. Measurement of<br />
antibody response to hepatitis B is<br />
not required either be<strong>for</strong>e or after<br />
this dose. (Local policies may vary).<br />
Refer to the Green Book <strong>for</strong> guidance<br />
regarding this detail and also<br />
occupational health risk.<br />
HBvaxPRO® 0, 1, and 6 months 16 years and over<br />
HBvaxPRO ® 0, 1, 2 and 12 months 16 years and over<br />
HBvaxPRO ® Paediatric 0, 1 & 6 months 0 <strong>–</strong> 15 years<br />
HBvaxPRO ® Paediatric 0, 1, 2, 12 months<br />
COMBINED <strong>vaccines</strong> (and schedules)<br />
0 <strong>–</strong> 15 years<br />
Hepatyrix® (Hepatitis A and typhoid) Single dose 15 years and over 6-12 months <strong>for</strong> hepatitis A<br />
(Currently not available)<br />
component (then hepatitis A booster<br />
VIATIM® (Hepatitis A and typhoid) Single dose 16 years and over given as a monovalent vaccine) and 3<br />
years <strong>for</strong> the typhoid component<br />
Twinrix Adult® (Hepatitis A and B) 0, 1, 6 months 16 years and over See in<strong>for</strong>mation about hepatitis A<br />
Twinrix Adult® 0, 7, 21, days and 12 months 18 years and over and hepatitis B regarding boosters<br />
Twinrix Paediatric® 0, 1, 6 months 1 <strong>–</strong> 15 years<br />
above<br />
Ambirix ® (Hepatitis A and B) 0 & 6-12 months 1 <strong>–</strong> 15 years<br />
Tetanus, polio & low dose diphtheria (<strong>for</strong> <strong>travel</strong> purposes)<br />
Revaxis® 1 dose if risk at destination and UK<br />
schedule complete more than 10 years ago <strong>–</strong> see<br />
Green Book p372<br />
From 6 years - <strong>for</strong> <strong>travel</strong><br />
purposes expect to give<br />
older than this<br />
10 years if risk at destination and risk<br />
of immunoglobulin not being<br />
available<br />
This chart was designed & created <strong>by</strong> <strong>Jane</strong> <strong>Chiodini</strong> ©Travel Health Training Ltd. Updated 20.03.13
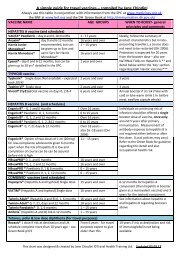
MENINGOCOCCAL vaccine<br />
ACWY Vax® Single dose (polysaccharide vaccine) 2 years and over 3-yearly <strong>for</strong> pilgrims to Hajj or<br />
Menveo Umrah. The need <strong>for</strong> timing of a<br />
booster of Menveo and Nimenrix has<br />
not yet been established <strong>–</strong> see Green<br />
Book update (Nimenrix not yet<br />
added) and SPCs<br />
▼® Single dose (conjugate vaccine) 2 years<br />
Nimenrix ▼ Single dose (conjugate vaccine)<br />
RABIES vaccine (and schedules)<br />
1 year<br />
Rabies vaccine BP 0, 7 and 28 days Any age but careful risk New Green Book chapter due <strong>–</strong> see<br />
Rabipur® 0, 7, 21 or 28 days<br />
YELLOW FEVER vaccine<br />
assessment under 1 year JCVI Oct. 2010 minutes at<br />
http://www.dh.gov.uk/ab/JCVI/DH_1<br />
07556 & see TRAVAX<br />
Stamaril® Single dose<br />
CHOLERA vaccine<br />
Over 9 months 10 years <strong>–</strong> certificate valid 10 days<br />
after vaccine administered<br />
Dukoral® Oral vaccine. 2 doses, 1 wk apart from 2 years and over 2 yrs in age 6 to adult<br />
6yrs of age. 3 doses, minimum 1 wk apart in 2 <strong>–</strong><br />
6 months in 2 <strong>–</strong> 6 year olds<br />
6 year olds<br />
JAPANESE ENCEPHALITIS vaccine<br />
NBM 1 hr be<strong>for</strong>e & after vaccine<br />
IXIARO ®▼ 0 and 28 days <strong>for</strong> all age groups. From 2 months 1 year if at continuous risk<br />
0.5ml dose <strong>for</strong> adults & 3 yr to < 18 yr age group<br />
All others, boost at 12-24 months<br />
0.25ml <strong>for</strong> 2 month to < 3yr age group See SPC<br />
after the recommended primary<br />
<strong>for</strong> specific instructions)<br />
immunisation, prior to potential reexposure<br />
to Japanese encephalitis<br />
TICK BORNE ENCEPHALITIS vaccine<br />
Tico-Vac® 3 doses of 0.5ml on day 0, 1-3 months<br />
after 1 st dose, 5-12 months after 2 nd dose<br />
Tico-Vac® Junior (0.25ml) 3 doses <strong>–</strong> same dosing<br />
schedule as adult Tico-Vac®<br />
16 years and over Booster every 3 years after initial 3<br />
1 year to below 16 years<br />
of age<br />
dose course is individual continues to<br />
be at risk. For rapid short-term<br />
protection <strong>–</strong> 2 nd dose can be given 2<br />
weeks after 1 st dose (giving at least<br />
90% protection) <strong>–</strong> see ‘Green Book’<br />
KEY<br />
* Within the Summary of Product Characteristics (SPC)<br />
** The Green Book (2006) refers to all hep A products, so the 20 year protection also applies to the<br />
combined products and paediatric hepatitis A <strong>vaccines</strong>. Also see the NaTHNaC FAQ document on hepatitis<br />
A <strong>for</strong> more exact detail http://www.nathnac.org/pro/misc/hepA.htm<br />
***SPC <strong>for</strong> Havrix Monodose and Havrix Junior Monodose April 2012 states ‘Current recommendations do<br />
not support the need <strong>for</strong> further booster vaccination among immunocompetent subjects after a 2 dose<br />
vaccination course’<br />
Sources of In<strong>for</strong>mation <strong>for</strong> this table taken from:<br />
Department of Health (2006) Immunisation Against Infectious Diseases <strong>–</strong> The Green Book<br />
http://immunisation.dh.gov.uk/category/the-green-book/ and subsequent update patches and revised<br />
chapters found on this website.<br />
Electronic Medicines Compendium www.emc.medicines.org.uk and also www.smpsd.co.uk,<br />
www.<strong>vaccines</strong>.co.uk, www.bnf.org<br />
IMPORTANT <strong>–</strong> Where there is a difference between the Green Book and the SPC, the Green Book should be<br />
followed <strong>–</strong> ref. Chapter 4, page 25<br />
PLEASE MAKE SURE YOU CHECK YOU ARE ALWAYS USING THE LATEST VERSION OF THIS CHART<br />
found at www.janechiodini.co.uk<br />
This chart was designed & created <strong>by</strong> <strong>Jane</strong> <strong>Chiodini</strong> ©Travel Health Training Ltd. Updated 20.03.13