Determination of Mechanism of Flock Sediment Formation in Tea ...
Determination of Mechanism of Flock Sediment Formation in Tea ...
Determination of Mechanism of Flock Sediment Formation in Tea ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
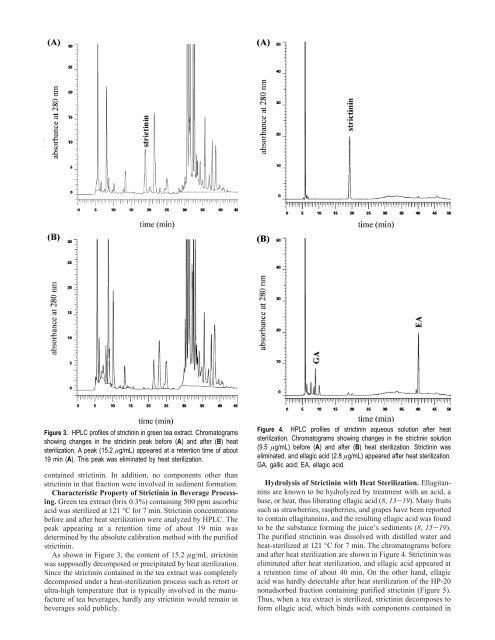
Figure 3. HPLC pr<strong>of</strong>iles <strong>of</strong> strict<strong>in</strong><strong>in</strong> <strong>in</strong> green tea extract. Chromatograms<br />
show<strong>in</strong>g changes <strong>in</strong> the strict<strong>in</strong><strong>in</strong> peak before (A) and after (B) heat<br />
sterilization. A peak (15.2 µg/mL) appeared at a retention time <strong>of</strong> about<br />
19 m<strong>in</strong> (A). This peak was elim<strong>in</strong>ated by heat sterilization.<br />
conta<strong>in</strong>ed strict<strong>in</strong><strong>in</strong>. In addition, no components other than<br />
strict<strong>in</strong><strong>in</strong> <strong>in</strong> that fraction were <strong>in</strong>volved <strong>in</strong> sediment formation.<br />
Characteristic Property <strong>of</strong> Strict<strong>in</strong><strong>in</strong> <strong>in</strong> Beverage Process<strong>in</strong>g.<br />
Green tea extract (brix 0.3%) conta<strong>in</strong><strong>in</strong>g 500 ppm ascorbic<br />
acid was sterilized at 121 °C for 7 m<strong>in</strong>. Strict<strong>in</strong><strong>in</strong> concentrations<br />
before and after heat sterilization were analyzed by HPLC. The<br />
peak appear<strong>in</strong>g at a retention time <strong>of</strong> about 19 m<strong>in</strong> was<br />
determ<strong>in</strong>ed by the absolute calibration method with the purified<br />
strict<strong>in</strong><strong>in</strong>.<br />
As shown <strong>in</strong> Figure 3, the content <strong>of</strong> 15.2 µg/mL strict<strong>in</strong><strong>in</strong><br />
was supposedly decomposed or precipitated by heat sterilization.<br />
S<strong>in</strong>ce the strict<strong>in</strong><strong>in</strong> conta<strong>in</strong>ed <strong>in</strong> the tea extract was completely<br />
decomposed under a heat-sterilization process such as retort or<br />
ultra-high temperature that is typically <strong>in</strong>volved <strong>in</strong> the manufacture<br />
<strong>of</strong> tea beverages, hardly any strict<strong>in</strong><strong>in</strong> would rema<strong>in</strong> <strong>in</strong><br />
beverages sold publicly.<br />
Figure 4. HPLC pr<strong>of</strong>iles <strong>of</strong> strict<strong>in</strong><strong>in</strong> aqueous solution after heat<br />
sterilization. Chromatograms show<strong>in</strong>g changes <strong>in</strong> the strict<strong>in</strong><strong>in</strong> solution<br />
(9.5 µg/mL) before (A) and after (B) heat sterilization. Strict<strong>in</strong><strong>in</strong> was<br />
elim<strong>in</strong>ated, and ellagic acid (2.8 µg/mL) appeared after heat sterilization.<br />
GA, gallic acid; EA, ellagic acid.<br />
Hydrolysis <strong>of</strong> Strict<strong>in</strong><strong>in</strong> with Heat Sterilization. Ellagitann<strong>in</strong>s<br />
are known to be hydrolyzed by treatment with an acid, a<br />
base, or heat, thus liberat<strong>in</strong>g ellagic acid (8, 13-19). Many fruits<br />
such as strawberries, raspberries, and grapes have been reported<br />
to conta<strong>in</strong> ellagitann<strong>in</strong>s, and the result<strong>in</strong>g ellagic acid was found<br />
to be the substance form<strong>in</strong>g the juice’s sediments (8, 15-19).<br />
The purified strict<strong>in</strong><strong>in</strong> was dissolved with distilled water and<br />
heat-sterilized at 121 °C for 7 m<strong>in</strong>. The chromatograms before<br />
and after heat sterilization are shown <strong>in</strong> Figure 4. Strict<strong>in</strong><strong>in</strong> was<br />
elim<strong>in</strong>ated after heat sterilization, and ellagic acid appeared at<br />
a retention time <strong>of</strong> about 40 m<strong>in</strong>. On the other hand, ellagic<br />
acid was hardly detectable after heat sterilization <strong>of</strong> the HP-20<br />
nonadsorbed fraction conta<strong>in</strong><strong>in</strong>g purified strict<strong>in</strong><strong>in</strong> (Figure 5).<br />
Thus, when a tea extract is sterilized, strict<strong>in</strong><strong>in</strong> decomposes to<br />
form ellagic acid, which b<strong>in</strong>ds with components conta<strong>in</strong>ed <strong>in</strong>