MOURA Dinara Jaqueline et al.
MOURA Dinara Jaqueline et al.
MOURA Dinara Jaqueline et al.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
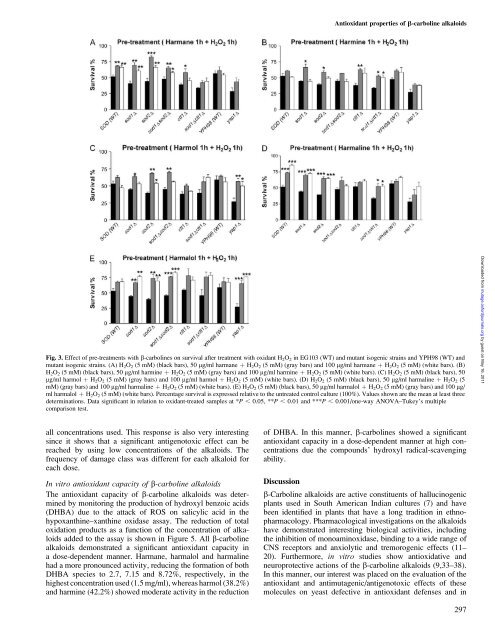
Fig. 3. Effect of pre-treatments with b-carbolines on surviv<strong>al</strong> after treatment with oxidant H 2O 2 in EG103 (WT) and mutant isogenic strains and YPH98 (WT) and<br />
mutant isogenic strains. (A) H2O2 (5 mM) (black bars), 50 lg/ml harmane þ H2O2 (5 mM) (gray bars) and 100 lg/ml harmane þ H2O2 (5 mM) (white bars). (B)<br />
H2O2 (5 mM) (black bars), 50 lg/ml harmine þ H2O2 (5 mM) (gray bars) and 100 lg/ml harmine þ H2O2 (5 mM) (white bars). (C) H2O2 (5 mM) (black bars), 50<br />
lg/ml harmol þ H2O2 (5 mM) (gray bars) and 100 lg/ml harmol þ H2O2 (5 mM) (white bars). (D) H2O2 (5 mM) (black bars), 50 lg/ml harm<strong>al</strong>ine þ H2O2 (5<br />
mM) (gray bars) and 100 lg/ml harm<strong>al</strong>ine þ H2O2 (5 mM) (white bars). (E) H2O2 (5 mM) (black bars), 50 lg/ml harm<strong>al</strong>ol þ H2O2 (5 mM) (gray bars) and 100 lg/<br />
ml harm<strong>al</strong>ol þ H 2O 2 (5 mM) (white bars). Percentage surviv<strong>al</strong> is expressed relative to the untreated control culture (100%). V<strong>al</strong>ues shown are the mean at least three<br />
d<strong>et</strong>erminations. Data significant in relation to oxidant-treated samples at *P , 0.05, **P , 0.01 and ***P , 0.001/one-way ANOVA–Tukey’s multiple<br />
comparison test.<br />
<strong>al</strong>l concentrations used. This response is <strong>al</strong>so very interesting<br />
since it shows that a significant antigenotoxic effect can be<br />
reached by using low concentrations of the <strong>al</strong>k<strong>al</strong>oids. The<br />
frequency of damage class was different for each <strong>al</strong>k<strong>al</strong>oid for<br />
each dose.<br />
In vitro antioxidant capacity of b-carboline <strong>al</strong>k<strong>al</strong>oids<br />
The antioxidant capacity of b-carboline <strong>al</strong>k<strong>al</strong>oids was d<strong>et</strong>ermined<br />
by monitoring the production of hydroxyl benzoic acids<br />
(DHBA) due to the attack of ROS on s<strong>al</strong>icylic acid in the<br />
hypoxanthine–xanthine oxidase assay. The reduction of tot<strong>al</strong><br />
oxidation products as a function of the concentration of <strong>al</strong>k<strong>al</strong>oids<br />
added to the assay is shown in Figure 5. All b-carboline<br />
<strong>al</strong>k<strong>al</strong>oids demonstrated a significant antioxidant capacity in<br />
a dose-dependent manner. Harmane, harm<strong>al</strong>ol and harm<strong>al</strong>ine<br />
had a more pronounced activity, reducing the formation of both<br />
DHBA species to 2.7, 7.15 and 8.72%, respectively, in the<br />
highest concentration used (1.5 mg/ml), whereas harmol (38.2%)<br />
and harmine (42.2%) showed moderate activity in the reduction<br />
Antioxidant properties of b-carboline <strong>al</strong>k<strong>al</strong>oids<br />
of DHBA. In this manner, b-carbolines showed a significant<br />
antioxidant capacity in a dose-dependent manner at high concentrations<br />
due the compounds’ hydroxyl radic<strong>al</strong>-scavenging<br />
ability.<br />
Discussion<br />
b-Carboline <strong>al</strong>k<strong>al</strong>oids are active constituents of h<strong>al</strong>lucinogenic<br />
plants used in South American Indian cultures (7) and have<br />
been identified in plants that have a long tradition in <strong>et</strong>hnopharmacology.<br />
Pharmacologic<strong>al</strong> investigations on the <strong>al</strong>k<strong>al</strong>oids<br />
have demonstrated interesting biologic<strong>al</strong> activities, including<br />
the inhibition of monoaminoxidase, binding to a wide range of<br />
CNS receptors and anxiolytic and tremorogenic effects (11–<br />
20). Furthermore, in vitro studies show antioxidative and<br />
neuroprotective actions of the b-carboline <strong>al</strong>k<strong>al</strong>oids (9,33–38).<br />
In this manner, our interest was placed on the ev<strong>al</strong>uation of the<br />
antioxidant and antimutagenic/antigenotoxic effects of these<br />
molecules on yeast defective in antioxidant defenses and in<br />
297<br />
Downloaded from<br />
mutage.oxfordjourn<strong>al</strong>s.org by guest on May 16, 2011