Lecture 1C: Examples of Chemical Equilibrium Calculation
Lecture 1C: Examples of Chemical Equilibrium Calculation
Lecture 1C: Examples of Chemical Equilibrium Calculation
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Lecture</strong> <strong>1C</strong>: <strong>Examples</strong> <strong>of</strong> <strong>Chemical</strong> <strong>Equilibrium</strong> <strong>Calculation</strong><br />
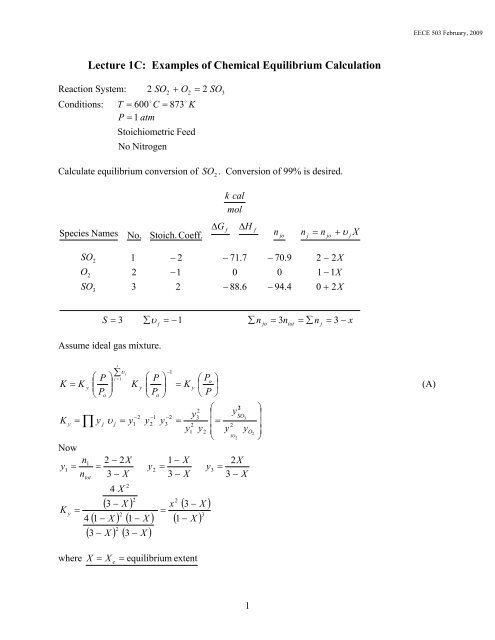
Reaction System: 2 SO 2 + O2<br />
= 2 SO3<br />
o<br />
o<br />
Conditions: T = 600 C = 873 K<br />
P = 1 atm<br />
Stoichiometric<br />
Feed<br />
No Nitrogen<br />
Calculate equilibrium conversion <strong>of</strong> SO 2 . Conversion <strong>of</strong> 99% is desired.<br />
Species Names<br />
SO<br />
O2<br />
SO<br />
2<br />
3<br />
S = 3<br />
No.<br />
Assume ideal gas mixture.<br />
K = K<br />
K<br />
y<br />
=<br />
y<br />
∏<br />
Now<br />
n<br />
y1<br />
=<br />
n<br />
K y<br />
=<br />
1<br />
tot<br />
⎛<br />
⎜<br />
⎝<br />
P<br />
P<br />
y<br />
j<br />
o<br />
s<br />
⎞<br />
∑<br />
j = 1<br />
⎟<br />
⎠<br />
υ<br />
j<br />
1<br />
2<br />
3<br />
υ = y<br />
2 − 2X<br />
=<br />
3 − X<br />
j<br />
K<br />
y<br />
−2<br />
1<br />
Stoich. Coeff.<br />
∑υ<br />
= −1<br />
⎛<br />
⎜<br />
⎝<br />
y<br />
j<br />
P<br />
P<br />
−1<br />
2<br />
y<br />
2 ( 3 − X )<br />
2 ( 1 − X ) ( 1 − X )<br />
2 ( 3 − X ) ( 3 − X )<br />
4<br />
4 X<br />
2<br />
2<br />
o<br />
⎞<br />
⎟<br />
⎠<br />
y<br />
− 2<br />
−1<br />
−1<br />
−2<br />
3<br />
2<br />
= K<br />
=<br />
1 −<br />
=<br />
3 −<br />
y<br />
X<br />
X<br />
y<br />
y<br />
⎛<br />
⎜<br />
⎝<br />
P<br />
2<br />
3<br />
2<br />
1 y2<br />
P<br />
∆G<br />
o<br />
⎞<br />
⎟<br />
⎠<br />
k cal<br />
mol<br />
f<br />
∆H<br />
− 71.<br />
7<br />
0<br />
− 88.<br />
6<br />
⎛<br />
⎜ y<br />
⎜=<br />
2<br />
⎜ y<br />
⎝<br />
y<br />
( )<br />
( ) 3<br />
2<br />
3 − X<br />
1 − X<br />
where = equilibrium<br />
extent<br />
X X<br />
= e<br />
=<br />
x<br />
3<br />
SO<br />
2<br />
2<br />
SO<br />
3<br />
y<br />
2X<br />
=<br />
3 − X<br />
∑ n<br />
1<br />
O<br />
2<br />
f<br />
⎞<br />
⎟<br />
⎟<br />
⎠<br />
jo<br />
n<br />
jo<br />
− 70.<br />
9<br />
0<br />
− 94.<br />
4<br />
= 3n<br />
tot<br />
n<br />
j<br />
= n<br />
= ∑ n<br />
2 − 2X<br />
1 − 1X<br />
0 + 2X<br />
j<br />
jo<br />
+ υ X<br />
j<br />
= 3 − x<br />
EECE 503 February, 2009<br />
(A)
From (A)<br />
x<br />
( )<br />
( ) ⎟ 2<br />
3 − x ⎛ P ⎞<br />
= K<br />
⎜<br />
3 873<br />
1 − x P<br />
Calculate K 298 at 298 K<br />
∆ o<br />
K<br />
G r<br />
298<br />
=<br />
= e<br />
⎝<br />
o<br />
⎠<br />
( − ) × ( − 71.<br />
7)<br />
+ ( −1)<br />
× ( 0)<br />
+ 2 × ( − 88.<br />
6)<br />
2 = − 33<br />
o<br />
∆Gr<br />
−<br />
RT<br />
o<br />
= e<br />
Calculate ∆ H r , K873<br />
∆<br />
H o<br />
r<br />
=<br />
−33,<br />
800<br />
−<br />
1.<br />
987 × 298<br />
= e<br />
57.<br />
08<br />
= 6 . 17 × 10<br />
( − ) × ( − 70.<br />
9)<br />
+ ( 1)<br />
× ( 0)<br />
+ 2 × ( − 94.<br />
4)<br />
2 = − 47<br />
24<br />
2<br />
. 0<br />
. 8<br />
k cal<br />
mol<br />
k cal<br />
mol<br />
Assume for simplicity (in order to find a first estimate for equilibrium conditions) that<br />
o<br />
∆ H ≈ const ≈ ∆H<br />
r<br />
Then<br />
d ln<br />
K ∆ H<br />
=<br />
dT RT<br />
⎛ K<br />
l n<br />
⎜<br />
⎝ K<br />
K<br />
T<br />
T<br />
298<br />
= K<br />
o<br />
r<br />
2<br />
⎞ ∆H<br />
⎟ =<br />
⎠ R<br />
298<br />
e<br />
r<br />
; and at T = T = 298,<br />
K = K<br />
o<br />
r<br />
⎛<br />
⎜<br />
⎝<br />
1<br />
298<br />
o<br />
∆H<br />
r ⎛ 1 1 ⎞<br />
⎜ − ⎟<br />
R ⎝ 298 T ⎠<br />
1<br />
−<br />
T<br />
47,<br />
000 ⎛ 1<br />
1.<br />
987 ⎝ 298<br />
57.<br />
08<br />
K 873 = e − ⎜ − ⎟ =<br />
K 873 = 121.<br />
78<br />
⎞<br />
⎟<br />
⎠<br />
o<br />
1 ⎞<br />
873 ⎠<br />
e<br />
4.<br />
80<br />
Note the dramatic drop in K with temperature due to the exothermocity <strong>of</strong> the reaction<br />
( ∆H r < 0)<br />
. While equilibrium would have been all the way to the right at 298 K we cannot<br />
operate at such conditions because the rate is too low.<br />
298<br />
(B)
Let us see what are the equilibrium limitations at 873 K.<br />
Solve by trial and error equation (B) for equilibrium extent X.<br />
2 ( 3 − X )<br />
3 ( 1 − X )<br />
X ⎛ o<br />
= K 873<br />
⎜<br />
⎝<br />
P<br />
P<br />
⎞<br />
⎟<br />
⎠<br />
−1<br />
P<br />
where K 873 = 121.<br />
78;<br />
= 1<br />
P<br />
Rearrange to use Newton-Raphson procedure<br />
o<br />
( ) ( ) ( ) 3<br />
2<br />
x = 0 = X 3 − X − 121.<br />
78 1 − X<br />
( ) ( ) ( ) 2<br />
2<br />
φ x = 2X 3 − X − X + 3 × 121.<br />
78 1 X<br />
n = X n<br />
φ ( X n )<br />
D φ ( X )<br />
φ<br />
D −<br />
X<br />
+ 1 (C)<br />
n<br />
Using a starting guess <strong>of</strong> X = 0.<br />
5 the Newton Raphson algorithm (C) yields:<br />
Iteration No n = 0<br />
Extent 0.5<br />
X = X e = 0.<br />
777<br />
( mol)<br />
1<br />
0.656<br />
Conversion <strong>of</strong> SO 2 is:<br />
nSO<br />
− n<br />
2,<br />
0 SO2<br />
2 −<br />
xSO<br />
=<br />
=<br />
2 nSO2<br />
0<br />
x = 0.<br />
777