CYTIDINE DIPHOSPHATE DIACYLGLYCEROL - Lipid Library
CYTIDINE DIPHOSPHATE DIACYLGLYCEROL - Lipid Library
CYTIDINE DIPHOSPHATE DIACYLGLYCEROL - Lipid Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Cytidine diphosphate diacylglycerol: structure, occurrence and biochemistry<br />
<strong>CYTIDINE</strong> <strong>DIPHOSPHATE</strong><br />
<strong>DIACYLGLYCEROL</strong><br />
STRUCTURE, OCCURRENCE AND BIOCHEMISTRY<br />
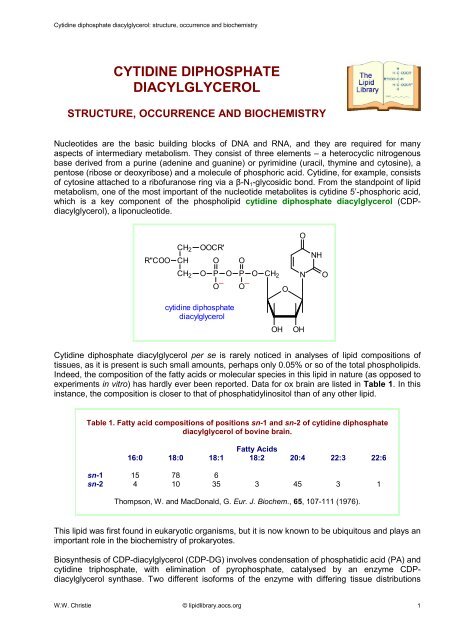
Nucleotides are the basic building blocks of DNA and RNA, and they are required for many<br />
aspects of intermediary metabolism. They consist of three elements – a heterocyclic nitrogenous<br />
base derived from a purine (adenine and guanine) or pyrimidine (uracil, thymine and cytosine), a<br />
pentose (ribose or deoxyribose) and a molecule of phosphoric acid. Cytidine, for example, consists<br />
of cytosine attached to a ribofuranose ring via a β-N1-glycosidic bond. From the standpoint of lipid<br />
metabolism, one of the most important of the nucleotide metabolites is cytidine 5’-phosphoric acid,<br />
which is a key component of the phospholipid cytidine diphosphate diacylglycerol (CDPdiacylglycerol),<br />
a liponucleotide.<br />
a<br />
R''COO<br />
CH 2<br />
CH<br />
CH 2<br />
OOCR'<br />
cytidine diphosphate<br />
diacylglycerol<br />
Cytidine diphosphate diacylglycerol per se is rarely noticed in analyses of lipid compositions of<br />
tissues, as it is present is such small amounts, perhaps only 0.05% or so of the total phospholipids.<br />
Indeed, the composition of the fatty acids or molecular species in this lipid in nature (as opposed to<br />
experiments in vitro) has hardly ever been reported. Data for ox brain are listed in Table 1. In this<br />
instance, the composition is closer to that of phosphatidylinositol than of any other lipid.<br />
Table 1. Fatty acid compositions of positions sn-1 and sn-2 of cytidine diphosphate<br />
diacylglycerol of bovine brain.<br />
Fatty Acids<br />
16:0 18:0 18:1 18:2 20:4 22:3 22:6<br />
sn-1 15 78 6<br />
sn-2 4 10 35 3 45 3 1<br />
Thompson, W. and MacDonald, G. Eur. J. Biochem., 65, 107-111 (1976).<br />
This lipid was first found in eukaryotic organisms, but it is now known to be ubiquitous and plays an<br />
important role in the biochemistry of prokaryotes.<br />
Biosynthesis of CDP-diacylglycerol (CDP-DG) involves condensation of phosphatidic acid (PA) and<br />
cytidine triphosphate, with elimination of pyrophosphate, catalysed by an enzyme CDPdiacylglycerol<br />
synthase. Two different isoforms of the enzyme with differing tissue distributions<br />
W.W. Christie © lipidlibrary.aocs.org<br />
O<br />
O<br />
O P O P O CH2 _ _<br />
O O O<br />
O<br />
N<br />
OH OH<br />
NH<br />
O<br />
a<br />
1
Cytidine diphosphate diacylglycerol: structure, occurrence and biochemistry<br />
have been found in animals, of which one has been has been designated the ‘housekeeping<br />
enzyme’ (CDS2) while the other is specialized for signal transduction (CDS1). A single enzyme is<br />
present in yeast, but multiple forms are found in plants such as Arabidopsis.<br />
a<br />
R''COO<br />
CH 2<br />
CH<br />
OOCR'<br />
O<br />
+ CTP<br />
R''COO<br />
CH2 O P OH<br />
_<br />
O<br />
+<br />
X<br />
CH2 O P O<br />
OH<br />
P O<br />
OH<br />
CH2 O<br />
phosphatidic acid cytidine diphosphate<br />
diacylglycerol<br />
The resulting CDP-diacylglycerol is utilized immediately for the synthesis of phosphatidylglycerol<br />
(PG), and thence cardiolipin (CL), and of phosphatidylinositol (PI) (see the appropriate web<br />
pages for these lipids). Turnover is very rapid and the pool of CDP-diacylglycrerol is always much<br />
smaller than that of the precursor phosphatidic acid. CDP-diacylglycerol for phosphatidylinositol<br />
synthesis is produced in the endoplasmic reticulum, whereas that for phosphatidylglycerol<br />
production is produced in the mitochondria. However, some transfer of the liponucleotide between<br />
organelles may be possible. In animals, phosphatidylcholine (PC), phosphatidyl ethanolamine (PE)<br />
and triacylglycerols (TG) are synthesised via the Kennedy pathway mainly with diacylglycerol as a<br />
key intermediate. CDP-diacylglycerol for phosphatidylinositol synthesis is produced in the<br />
endoplasmic reticulum, whereas that for phosphatidylglycerol production is produced in the<br />
mitochondria, by different isoforms of the CDP-diacylglycerol synthase.<br />
In fungi and prokaryotes, CDP-diacylglycerol is also the<br />
precursor for phosphatidylserine (PS). In yeast such<br />
as Saccharomyces cerevisiae, this can be a major route<br />
to phosphatidylethanolamine, which can in turn be<br />
converted via mono- and dimethylphosphatidylethanolamines<br />
(PME and PDE) to phosphatidylcholine,<br />
although the Kennedy pathway also functions. In the<br />
bacterium, Escherichia coli, CDP-diacylglycerols with<br />
both ribose and deoxyribose as the sugar component<br />
are produced, and both are utilized as substrates by<br />
phosphatidylserine and phosphatidylglycero-phosphate<br />
synthases.<br />
It is not known whether the final fatty acid composition of<br />
the lipid is a result of the specificity of the CDPdiacylglycerol<br />
synthase in selecting particular molecular<br />
species of phosphatidic acid, or whether remodelling<br />
occurs via deacylation/re-acylation reactions.<br />
Most studies of CDP-diacylglycerol have been<br />
concerned with its function as an intermediate in the<br />
biosynthesis of other lipids, and as such it is the first<br />
PC<br />
CDP-cho<br />
a<br />
step in a pathway that is very different from that for phosphatidylcholine and<br />
phosphatidylethanolamine. These also require nucleotides for their biosynthesis, but do not form<br />
liponucleotides as intermediates. Similarly, another nucleotide uridine 5-diphosphate(UDP)-hexose<br />
W.W. Christie © lipidlibrary.aocs.org<br />
CH 2<br />
CH<br />
OOCR'<br />
a<br />
O<br />
O<br />
CDP-DG<br />
PI<br />
PG<br />
PS<br />
CL<br />
CDP-DG<br />
pathway<br />
PE<br />
PME<br />
PDE<br />
O<br />
N<br />
OH OH<br />
PA<br />
CDP-Etn<br />
NH<br />
DG<br />
O<br />
a<br />
Kennedy<br />
pathway<br />
TG<br />
2
Cytidine diphosphate diacylglycerol: structure, occurrence and biochemistry<br />
(where hexose = glucose, galactose, etc) is required for the formation of glycolipids, including both<br />
the glycosyldiacylglycerols and sphingoglycolipids.<br />
The extent of the biological functions of CDP-diacylglycerol, other than as an intermediate in<br />
phospholipid biosynthesis, is only partly understood. However, CDP-diacylglycerol synthase is also<br />
a regulator of phospholipid metabolism, as it is believed to be the rate-limiting enzyme in<br />
phosphatidylinositol biosynthesis. In consequence, it has a role in the regulation of lipid-dependent<br />
signal transduction processes. Anti-cancer activities in vitro have been reported.<br />
Because it is such a minor component of tissues, isolation of cytidine diphosphate diacylglycerol<br />
appears to be a tedious task, involving ion-exchange column chromatography and thin-layer<br />
chromatography. The pyrophosphate bond is relatively labile and is very susceptible to alkaline<br />
hydrolysis. At natural tissue levels, even the more modern mass spectrometric methods do not<br />
appear to be sufficiently sensitive.<br />
Recommended Reading<br />
o Dowhan, W. CDP-diacylglycerol synthase of microorganisms. Biochim. Biophys. Acta, 1348, 157-165<br />
(1997).<br />
o Heacock, A.M. and Agranoff, B.W. CDP-diacylglycerol synthase from mammalian tissues. Biochim.<br />
Biophys. Acta, 1348, 166-172 (1997).<br />
o Vance, D.E. and Vance, J. (editors) Biochemistry of <strong>Lipid</strong>s, Lipoproteins and Membranes. 4 th Edition.<br />
(Elsevier, Amsterdam) (2002) – several chapters.<br />
William W. Christie<br />
Scottish Crop Research Institute (Mylnefield <strong>Lipid</strong> Analysis), Invergowrie, Dundee<br />
(DD2 5DA), Scotland<br />
Last updated: February 9 th , 2011<br />
W.W. Christie © lipidlibrary.aocs.org<br />
3