Lunesta Letter - Haymarket Media Group
Lunesta Letter - Haymarket Media Group
Lunesta Letter - Haymarket Media Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
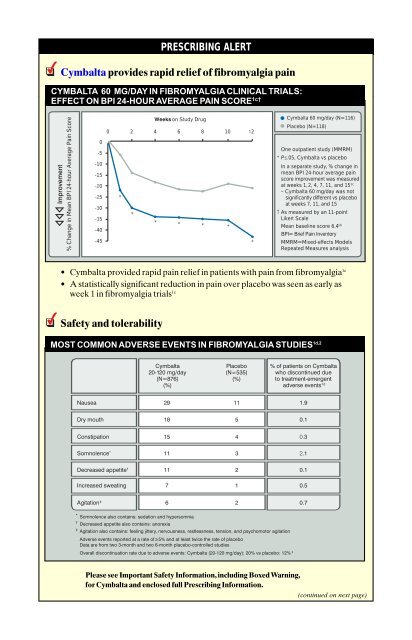
Cymbalta provides rapid relief of fibromyalgia pain<br />
• Cymbalta provided rapid pain relief in patients with pain from fibromyalgia 1c<br />
• A statistically significant reduction in pain over placebo was seen as early as<br />
week 1 in fibromyalgia trials 1c<br />
Safety and tolerability<br />
Nausea<br />
Dry mouth<br />
Constipation<br />
Somnolence *<br />
Decreased appetite †<br />
Increased sweating<br />
Agitation ‡<br />
PRESCRIBING ALERT<br />
CYMBALTA 60 MG/DAY IN FIBROMYALGIA CLINICAL TRIALS:<br />
EFFECT ON BPI 24-HOUR AVERAGE PAIN SCORE 1c†<br />
Improvement<br />
% Change in Mean BPI 24-hour Average Pain Score<br />
0<br />
-5<br />
-10<br />
-15<br />
-20<br />
-25<br />
-30<br />
-35<br />
-40<br />
-45<br />
Weeks on Study Drug<br />
0 2 4 6 8 10 12<br />
*<br />
*<br />
*<br />
MOST COMMON ADVERSE EVENTS IN FIBROMYALGIA STUDIES 1d,2<br />
Cymbalta<br />
20-120 mg/day<br />
(N=876)<br />
(%)<br />
29<br />
18<br />
15<br />
11<br />
11<br />
7<br />
6<br />
*<br />
Placebo<br />
(N=535)<br />
(%)<br />
*<br />
Somnolence also contains: sedation and hypersomnia<br />
†<br />
Decreased appetite also contains: anorexia<br />
‡<br />
Agitation also contains: feeling jittery, nervousness, restlessness, tension, and psychomotor agitation<br />
Adverse events reported at a rate of ≥5% and at least twice the rate of placebo<br />
Data are from two 3-month and two 6-month placebo-controlled studies<br />
Overall discontinuation rate due to adverse events: Cymbalta (20-120 mg/day): 20% vs placebo: 12% 2<br />
Please see Important Safety Information, including Boxed Warning,<br />
for Cymbalta and enclosed full Prescribing Information.<br />
*<br />
*<br />
11<br />
5<br />
4<br />
3<br />
2<br />
1<br />
2<br />
*<br />
Cymbalta 60 mg/day (N=116)<br />
Placebo (N=118)<br />
One outpatient study (MMRM)<br />
* P≤.05, Cymbalta vs placebo<br />
In a separate study, % change in<br />
mean BPI 24-hour average pain<br />
score improvement was measured<br />
at weeks 1, 2, 4, 7, 11, and 151c – Cymbalta 60 mg/day was not<br />
significantly different vs placebo<br />
at weeks 7, 11, and 15<br />
† As measured by an 11-point<br />
Likert Scale<br />
Mean baseline score 6.41b BPI= Brief Pain Inventory<br />
MMRM=Mixed-effects Models<br />
Repeated Measures analysis<br />
% of patients on Cymbalta<br />
who discontinued due<br />
to treatment-emergent<br />
adverse events 1d<br />
1.9<br />
0.1<br />
0.3<br />
2.1<br />
0.1<br />
0.5<br />
0.7<br />
(continued on next page)