APOPTOSIS: Programmed Cell Death APOPTOSIS
APOPTOSIS: Programmed Cell Death APOPTOSIS
APOPTOSIS: Programmed Cell Death APOPTOSIS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>APOPTOSIS</strong>: <strong>Programmed</strong> <strong>Cell</strong> <strong>Death</strong><br />
156:201 Principles of Molecular & <strong>Cell</strong> Biology<br />
October 24, 2003<br />
Reading: Lodish Chapter 22, pp. 924-930<br />
Discussion Group: October 27, 2003<br />
Paper: Sogame N, Kim M, Abrams JM. Drosophila p53 preserves genomic<br />
stability by regulating cell death. PNAS 100: 4697-4701, 2003.<br />
Jackie Bickenbach, PhD<br />
jackie-bickenbach@uiowa.edu<br />
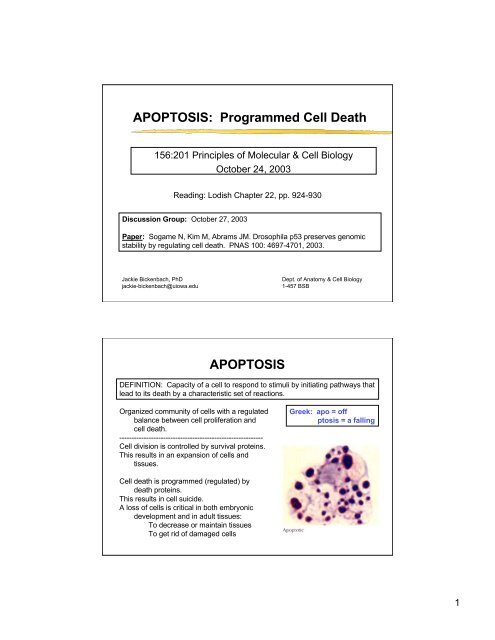
<strong>APOPTOSIS</strong><br />
Dept. of Anatomy & <strong>Cell</strong> Biology<br />
1-457 BSB<br />
DEFINITION: Capacity of a cell to respond to stimuli by initiating pathways that<br />
lead to its death by a characteristic set of reactions.<br />
Organized community of cells with a regulated<br />
balance between cell proliferation and<br />
cell death.<br />
-----------------------------------------------------------<br />
<strong>Cell</strong> division is controlled by survival proteins.<br />
This results in an expansion of cells and<br />
tissues.<br />
<strong>Cell</strong> death is programmed (regulated) by<br />
death proteins.<br />
This results in cell suicide.<br />
A loss of cells is critical in both embryonic<br />
development and in adult tissues:<br />
To decrease or maintain tissues<br />
To get rid of damaged cells<br />
Greek: apo = off<br />
ptosis = a falling<br />
1
Apoptosis is not the same as Necrosis<br />
Necrosis<br />
- cells are damaged<br />
- cells swell, then burst<br />
- triggers a destructive immune<br />
response<br />
- acute “messy” response<br />
Apoptosis<br />
- cells die neatly<br />
- cells do not damage neighbors<br />
- programmed, regulated response<br />
- no attraction of destructive immune cells<br />
- apoptotic cells are neatly cleared away<br />
(engulfed by macrophages or by neighboring cells)<br />
Regulated <strong>Cell</strong> <strong>Death</strong> During Development<br />
In frog development, an increase in thyroid<br />
hormone induces apoptosis in the<br />
cells in the tadpole tail.<br />
In mouse development, digits are formed<br />
only if cells in the web between each<br />
digit are programmed to die.<br />
2
Regulation of the number of nerve cells<br />
Binding of specific neurotrophins<br />
(NGF, BDNF, NT3) to their specific<br />
Trk receptor (receptor tyrosine<br />
kinases) produces survival signals for<br />
a specific class of neurons.<br />
Lodish figure 22-28<br />
suckling 9 hrs. post-suckling<br />
Only nerve cells that<br />
receive a survival signal<br />
live.<br />
Balances number of nerve<br />
cells to number of target<br />
receptors.<br />
Balance of cell<br />
proliferation with<br />
apoptosis in adult<br />
mammary tissues<br />
Ductal epithelial cells proliferate in response to<br />
hormones produced by birth of offspring.<br />
Sucking triggers milk production via oxytocin<br />
hormone produced in hypothalamus.<br />
When suckling stops, TGFb3 (brown stain in B)<br />
accumulates in ducts with undrained milk<br />
- triggers apoptosis (brown cells in C)<br />
- unneeded cells are eliminated<br />
- ducts regress and gland goes back to<br />
resting state<br />
3
Apoptosis: <strong>Programmed</strong><br />
(Regulated) <strong>Cell</strong> <strong>Death</strong><br />
Apoptosis is characterized by:<br />
Shrinking cells<br />
Chromatin compaction<br />
Degradation of chromosomal DNA<br />
Nuclear fragmentation<br />
Condensation of cytoplasm<br />
Blebbing<br />
<strong>Cell</strong> fragmentation<br />
<strong>Cell</strong> surface alterations cause rapid phagocytosis,<br />
which allows recycling of cellular proteins.<br />
Apoptosis can be triggered by internal or external<br />
stimuli.<br />
Apoptosis Movie<br />
Lodish Figure 22-26<br />
4
Sigma-Aldrich<br />
There are<br />
multiple<br />
apoptotic<br />
pathways<br />
In all pathways control<br />
of apoptosis is<br />
regulated by a balance<br />
between activation and<br />
inhibition.<br />
It is thought that all cells<br />
have the capacity to<br />
apoptose.<br />
Thus, the critical<br />
determinant of whether<br />
a cell lives or dies may<br />
depend upon its ability<br />
to activate or inhibit<br />
these apoptotic<br />
pathways.<br />
Fas-Fas Ligand Triggers Apoptosis<br />
Fas receptor on a target cell is<br />
activated with the FasL protein.<br />
(Fas is related to TNF receptor,<br />
FasL is related to TNF.<br />
FasL protein is on an activating cell<br />
plasma membrane.<br />
Upon interaction with FasL, Fas<br />
trimerizes.<br />
Fas has a cytoplasmic domain often<br />
called the “death domain”.<br />
The death domain is involved with<br />
protein-protein interactions.<br />
e.g. Fas-FADD-capsase 8<br />
Note: Fas and FasL can be on<br />
different cells or one the same<br />
cell.<br />
5
Caspases:<br />
family of cysteine aspartate proteases<br />
Caspases have a catalytic cysteine and cleave<br />
their targets at an asparate.<br />
2 subfamilies:<br />
Caspase 1 involved in inflammation.<br />
Caspases 3, 6-10 involved in apoptosis.<br />
All are synthesized as inactive procaspases.<br />
All, but the initial caspase in a cascade, are<br />
activated by proteolytic cleaving by another<br />
caspase family member. (First caspase in<br />
the cascade is autocleaved)<br />
ÿ Prodomain is cleaved off<br />
ÿ Then caspase is cleaved into small<br />
and large subunits<br />
ÿ Cleaved subunits associate to form<br />
active caspase (likely a tetramer)<br />
Caspase Cascade<br />
Caspase Cascade:<br />
1. Small number of caspases<br />
activated<br />
2. Rapid autocleaving of more<br />
caspases.<br />
3. These initiator caspases also<br />
activate other caspases by<br />
cleaving procaspases<br />
4. These then activate additional<br />
caspases (Effector Caspases)<br />
5. The latter caspases cleave other<br />
cellular proteins<br />
e.g. cellular proteins to release<br />
DNAse, nuclear lamin to<br />
release DNA<br />
6
Caspases first discovered in c. elegans<br />
C. elegans has one effector caspase, CED-3.<br />
Humans have 15 caspases, 6 involved in apoptosis (3, 6-10).<br />
Three types of proteins are involved in regulation of apoptosis:<br />
Regulator proteins can either promote or suppress apoptosis.<br />
(CED-9 and Bcl-2 are suppressors)<br />
Adapter proteins coordinate the action of regulator and effector proteins to<br />
control apoptosis by interacting with both types of proteins.<br />
In the absence of trophic factors, they promote activation of effectors<br />
In the presence of trophic factors, they are inhibited by regulators.<br />
Activation of the<br />
Mitochondrial<br />
Caspase Pathway<br />
The caspase pathway moves from the<br />
cell membrane to the mitochondrial<br />
membrane via caspase 8.<br />
Caspase 8 cleaves Bid to release a Cterminal<br />
domain that translocates to<br />
the mitochondria.<br />
Bid is a member of the Bcl2 family and<br />
acts to cause the mitochondria to<br />
release cytochrome c.<br />
Mitochondria are also involved in the<br />
caspase cascade in c.elegans.<br />
Sigma-Aldrich<br />
Lodish<br />
Figure 22-31<br />
vertebrates<br />
c.elegans<br />
7
Mitochondria’s Roles in Apoptosis<br />
Pro-apoptotic role<br />
<strong>Cell</strong>s are damaged or stressed.<br />
Mitochondria are induced to release<br />
cytochrome c (electron carrier)<br />
Binds Apaf-1 (adaptor protein)<br />
Aggregates procaspase-9 proteins<br />
They cleave each other<br />
Release active caspase 9<br />
Activates downstream effector<br />
caspases 3,6,7<br />
Anti-apoptotic role<br />
Proteolytic activity of caspase 9 can<br />
be inhibited by IAP proteins.<br />
Proteins that antagonize IAPs may<br />
be released by mitochondria.<br />
Inhibitors of<br />
Apoptosis<br />
Sigma-Aldrich<br />
Apoptosis can be inhibited at stages<br />
catalyzed by the later caspases.<br />
IAP (inhibitor of apoptosis) Family<br />
bind some procaspases<br />
bind some caspases<br />
In order for apoptosis to proceed, IAPs<br />
must be blocked.<br />
When mitochondria release cytochrome<br />
c, they also release a protein<br />
(diablo) that blocks IAPs<br />
8
Bcl family members play roles as inhibitors<br />
and as promoters of apoptosis<br />
Bcl family<br />
<strong>Death</strong> Inhibitors<br />
Bcl-2 and Bcl-X block<br />
cytochrome c release<br />
Promoters of Apoptosis<br />
Bad--inhibits death inhibitors<br />
Bax, Bak, Bim, & Bid stimulate<br />
release of cytochrome c<br />
Caspase Activation vs.Inhibition<br />
Lodish Figure 22-32<br />
9
Mechanisms of Ageing and Development 123: 245-260, 2002.<br />
Mutations in<br />
apoptotic genes<br />
may extend life<br />
DAF-2 (c. elegans)<br />
Similar to IFG-I (mammals)<br />
Controls daur larva phase<br />
Animals with DAF-2 mutations<br />
Increase AGE-1 (PI3K)<br />
Increase wt AKT-1 (Akt)<br />
Increase IKK<br />
Results:<br />
• No cell death in the worm<br />
• Worms live twice as long<br />
Is there a switch between apoptosis and<br />
proliferation?<br />
J. Clin., Invest. 109: 437-442, 2002.<br />
FLIP<br />
Diverts molecules activated by<br />
Fas-FasL from caspase cascade<br />
to proliferative pathway<br />
Contains 2 DED (similar to<br />
caspase 8)<br />
Associates with<br />
Raf-1 (ERK) -> proliferation<br />
TRAF-1 (NF-kB) -> differentiation<br />
Thus cells with FLIP resist<br />
caspase -8 apoptosis and might<br />
be stimulated to proliferate<br />
instead.<br />
10
Apoptosis Movie<br />
References for Apoptosis<br />
1. Earnshaw et al (1999) “Mammalian Caspases: structure, activation,<br />
substrates and functions during apoptosis” Ann. Rev Biochem. 68, 383.<br />
2. Rhind and Russell (1998) “ Mitotic DNA damage and replication checkpoints<br />
in yeast” Curr. Opin. <strong>Cell</strong> Biol. 10, 749.<br />
3. Budihardjo, I., et al., Biochemical pathways of caspase activation during<br />
apoptosis. Annu. Rev. <strong>Cell</strong> Dev. Biol., 15, 269-290 (1999).<br />
4. Adrain, C., and Martin, S.J., The mitochondrial apoptosome: a killer<br />
unleashed by the cytochrome seas. Trends Biochem. Sci., 26, 390-397<br />
(2001).<br />
5. References listed at the end of Lodish Chapter 22.<br />
11