- 1 - CHEMISTRY 2 REVIEW: MIDTERM Everything Energy Matter ...
- 1 - CHEMISTRY 2 REVIEW: MIDTERM Everything Energy Matter ...
- 1 - CHEMISTRY 2 REVIEW: MIDTERM Everything Energy Matter ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
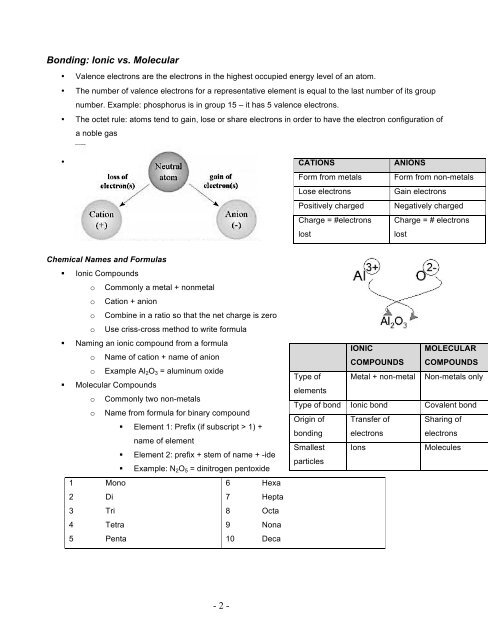
Bonding: Ionic vs. Molecular<br />
• Valence electrons are the electrons in the highest occupied energy level of an atom.<br />
• The number of valence electrons for a representative element is equal to the last number of its group<br />
number. Example: phosphorus is in group 15 – it has 5 valence electrons.<br />
• The octet rule: atoms tend to gain, lose or share electrons in order to have the electron configuration of<br />
•<br />
a noble gas<br />
Chemical Names and Formulas<br />
§ Ionic Compounds<br />
o Commonly a metal + nonmetal<br />
o Cation + anion<br />
o Combine in a ratio so that the net charge is zero<br />
o Use criss-cross method to write formula<br />
§ Naming an ionic compound from a formula<br />
o Name of cation + name of anion<br />
o Example Al2O3 = aluminum oxide<br />
§ Molecular Compounds<br />
o Commonly two non-metals<br />
o Name from formula for binary compound<br />
§ Element 1: Prefix (if subscript > 1) +<br />
name of element<br />
§ Element 2: prefix + stem of name + -ide<br />
§ Example: N2O5 = dinitrogen pentoxide<br />
1 Mono 6 Hexa<br />
2 Di 7 Hepta<br />
3 Tri 8 Octa<br />
4 Tetra 9 Nona<br />
5 Penta 10 Deca<br />
- 2 -<br />
CATIONS ANIONS<br />
Form from metals Form from non-metals<br />
Lose electrons Gain electrons<br />
Positively charged Negatively charged<br />
Charge = #electrons<br />
lost<br />
Type of<br />
elements<br />
IONIC<br />
Charge = # electrons<br />
lost<br />
COMPOUNDS<br />
MOLECULAR<br />
COMPOUNDS<br />
Metal + non-metal Non-metals only<br />
Type of bond Ionic bond Covalent bond<br />
Origin of<br />
bonding<br />
Smallest<br />
particles<br />
Transfer of<br />
electrons<br />
Sharing of<br />
electrons<br />
Ions Molecules