- 1 - CHEMISTRY 2 REVIEW: MIDTERM Everything Energy Matter ...
- 1 - CHEMISTRY 2 REVIEW: MIDTERM Everything Energy Matter ...
- 1 - CHEMISTRY 2 REVIEW: MIDTERM Everything Energy Matter ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Flame Test<br />
• A flame test can be used to identify a METAL.<br />
• An electron falling from an excited state will release energy in the<br />
form of colored light.<br />
• The color of the light depends upon the energy of the transition.<br />
o Red – lowest energy<br />
o Violet – highest energy<br />
Counting Atoms<br />
1. The symbol of an element represents 1 atom<br />
Example: Na = 1 atom of sodium<br />
2. A subscript indicates the number of atoms of a particular<br />
element<br />
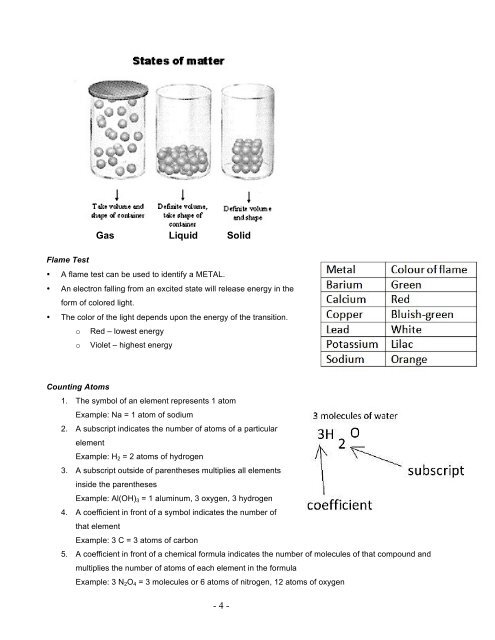
Gas<br />
Example: H2 = 2 atoms of hydrogen<br />
3. A subscript outside of parentheses multiplies all elements<br />
inside the parentheses<br />
Example: Al(OH)3 = 1 aluminum, 3 oxygen, 3 hydrogen<br />
4. A coefficient in front of a symbol indicates the number of<br />
that element<br />
Liquid<br />
Example: 3 C = 3 atoms of carbon<br />
5. A coefficient in front of a chemical formula indicates the number of molecules of that compound and<br />
multiplies the number of atoms of each element in the formula<br />
Example: 3 N2O4 = 3 molecules or 6 atoms of nitrogen, 12 atoms of oxygen<br />
- 4 -<br />
Solid