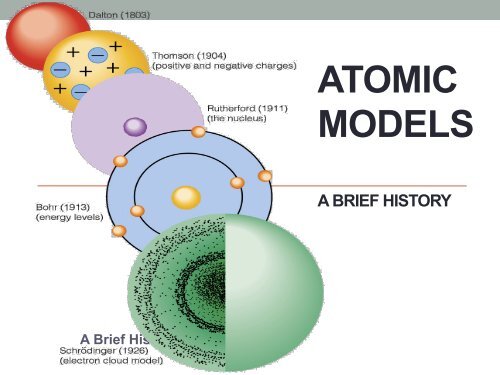
ATOMIC MODELS
ATOMIC MODELS
ATOMIC MODELS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
A Brief History<br />
<strong>ATOMIC</strong><br />
<strong>MODELS</strong><br />
A BRIEF HISTORY
THOMSON: PLUM PUDDING<br />
• Negative charges<br />
embedded in a ball of<br />
positive charge.<br />
• Problems<br />
• Could not explain<br />
scattering (Gold Foil)<br />
• Ionization
RUTHERFORD: NUCLEAR MODEL<br />
• Atom mostly empty space<br />
• Small dense core of positive<br />
charge (the nucleus)<br />
• Electrons in random orbits<br />
around the nucleus<br />
• Problems<br />
• Nuclear catastrophe: expected<br />
orbiting e- to crash into nucleus due<br />
to Columbic attraction<br />
• Could not explain chemical<br />
properties of the elements
BOHR: BOHR MODEL<br />
• Electrons<br />
• orbit nucleus in distinct, fixed orbits<br />
called energy levels<br />
• Energy level<br />
• a region around the nucleus where the<br />
electron is likely to be moving with a<br />
constant energy<br />
• Further away from the<br />
nucleus, the higher the<br />
energy
• Electrons may move to higher or excited energy states by<br />
absorbing a quanta of energy<br />
• Electrons may drop to lower energy states by releasing a<br />
quanta of energy, usually as light<br />
• Electrons may NOT exist between energy levels
BOHR MODEL<br />
• Explained<br />
• Stability of the atom<br />
• Atomic Emission Spectra<br />
• Periodic Chemical Behavior<br />
• Problems<br />
• Only explained observations for Hydrogen atoms
QUANTUM MECHANICAL MODEL<br />
• A mathematical model<br />
• Determines the allowed energies an<br />
electron can have<br />
• Defines a probability region around the<br />
nucleus where an electron is likely to be<br />
found
• Electron Cloud: probability region where an electron<br />
may be found 90% of the time<br />
• More dense closer to nucleus à higher probability
QUANTUM NUMBERS<br />
• Four quantum numbers fully describe an electron in the<br />
atom<br />
• No two electrons can be described by the same four quantum<br />
numbers
PRINCIPAL QUANTUM NUMBER<br />
• Symbol – n<br />
• Describes the main energy level occupied by the electron<br />
• A positive integer<br />
• Higher the energy level, the further from the nucleus<br />
• Max number of e- allowed in an energy level à 2n 2<br />
Energy Level Max Number of Electrons<br />
1 2<br />
2 8<br />
3 18<br />
4 32<br />
5 50
ORBITAL QUANTUM NUMBER<br />
• Describes an energy sublevel<br />
• Shape of the probability region (s, p, d, f)<br />
• Number of different sub-level types per energy level = n
ORBITAL QUANTUM NUMBER<br />
• Describes an energy sublevel<br />
• Shape of the probability region (s, p, d, f)<br />
• Number of different sub-level types per energy level = n<br />
Energy Level Allowed energy<br />
sublevels<br />
1 s 1<br />
2 s, p 2<br />
3 s, p, d 3<br />
4 s, p, d, f 4<br />
Number of<br />
sublevel
MAGNETIC QUANTUM NUMBER<br />
• Indicates the number and orientation of the atomic orbitals<br />
around the nucleus<br />
• The number of different ways each shape may position itself in<br />
space<br />
• Number of orbitals per energy level = n 2<br />
Shape Number of Orbitals<br />
s 1<br />
p 3<br />
d 5<br />
f 7
SPIN QUANTUM NUMBER<br />
• Indicates the orientation of the electron<br />
• One orbital may hold a max of two electrons<br />
• Electrons in same orbital must have opposite<br />
spins<br />
• Value of + ½ or – ½<br />
• Keeps any two e- from have the same four<br />
quantum numbers
QUESTIONS<br />
1. How is the QM model of the atom different from other<br />
models?<br />
2. What is an atomic orbital?<br />
3. How many orbitals in:<br />
• 3p sublevel - 3d sublevel<br />
• 2s sublevel - third energy level<br />
• 4f sublevel - 4p sublevel<br />
• How many sublevels in the following energy level<br />
• n=1, n=2, n=3, n=4