A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 2<br />
Table 2.7: Kinetic <strong>and</strong> thermodynamic parameters on the displacement reactions <strong>of</strong> complex<br />
1 <strong>and</strong> 2 with Hacac. 86<br />
Experiment kapp x 10 3<br />
1<br />
2<br />
(s -1 )<br />
T (K)<br />
0.3 ± 0.2 293<br />
0.6 ± 0.2 298<br />
1.2 ± 0.2 303<br />
2.1 ± 0.2 308<br />
3.1 ± 0.2 313<br />
0.1 ± 0.2 293<br />
0.2 ± 0.2 298<br />
0.5 ± 0.2 303<br />
0.9 ± 0.2 308<br />
1.5 ± 0.2 313<br />
Ea<br />
(kJ.mol -1 )<br />
39<br />
∆H ‡<br />
(kJ.mol -1 )<br />
∆S ‡<br />
(J.mol -1 .K -1 )<br />
90.3 ± 0.1 87.8 ± 0.1 -11 ± 4<br />
105.5 ± 0.1 102.9 ± 0.1 31 ± 4<br />
The kapp values were fit to the Arrhenius plot, kapp = Aexp(-Ea/RT), <strong>and</strong> the Ea values<br />
for the two processes were calculated (Table 2.7). The activation parameters, ∆H ‡<br />
<strong>and</strong> ∆S ‡ , were determined from the Eyring plot. The differences in the activation<br />
energy <strong>of</strong> the two processes were attributed to the more stable bond between the<br />
dbm lig<strong>and</strong> <strong>and</strong> the <strong>niobium</strong> atom in complex 2, through a delocalized π system due<br />
to the phenyl rings <strong>of</strong> the lig<strong>and</strong>, than in complex 1 with the dpm lig<strong>and</strong><br />
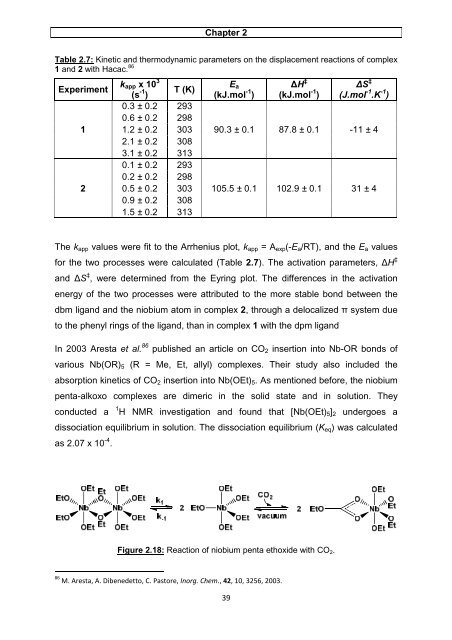
In 2003 Aresta et al. 86 published an article on CO2 insertion into Nb-OR bonds <strong>of</strong><br />
various Nb(OR)5 (R = Me, Et, allyl) <strong>complexes</strong>. Their <strong>study</strong> also included the<br />
absorption kinetics <strong>of</strong> CO2 insertion into Nb(OEt)5. As mentioned before, the <strong>niobium</strong><br />
penta-alkoxo <strong>complexes</strong> are dimeric in the <strong>solid</strong> <strong>state</strong> <strong>and</strong> in <strong>solution</strong>. They<br />
conducted a 1 H NMR investigation <strong>and</strong> found that [Nb(OEt)5]2 undergoes a<br />
dissociation equilibrium in <strong>solution</strong>. The dissociation equilibrium (Keq) was calculated<br />
as 2.07 x 10 -4 .<br />
Figure 2.18: Reaction <strong>of</strong> <strong>niobium</strong> penta ethoxide with CO2.<br />
86 M. Aresta, A. Dibenedetto, C. Pastore, Inorg. Chem., 42, 10, 3256, 2003.